Photodynamic therapy
Photodynamic therapy (PDT) involves the application of a so-called photosensitising chemical and subsequent irradiation with light of a suitable wavelength to convert the photosensitizer into a free radical (an atom/molecule/ion with an unpaired electron) which subsequently creates highly reactive oxygen radicals in the irradiated tissue, and only there (assuming that illumination can be exactly targeted at the diseased tissue / tumour).Free oxygen radicals are toxic for living cells and effectively kill a cell. PDT attempts to only create free radicals in irradiated diseased tissue and thus avoid some of the unwanted effects of other conventional, less selective treatments.
PDT is quite frequently used for the treatment of superficial skin cancers and other non-malignant skin lesions (such as actinic (‘solar’) keratoses or severe cases of acne). PDT is often provided as a service by dermatologists and uses a topical photoactive substance (ALA), used as a cream and applied to the area to be treated.
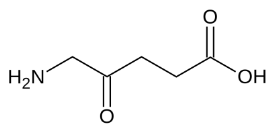
ALA is a small molecule, 5-aminolevulinic acid which is a precursor molecule in the biosynthesis of the actual photosensitiser.
For use in mucosal cancer of the head and neck in the UK PDT is only licensed for palliative use or when other standard strategies have failed or are not possible. PDT then involves an intravenous injection of a photosensitizer (temoporfin) about 4 days before illuminating the tumour either with superficial or interstitial red light laser.
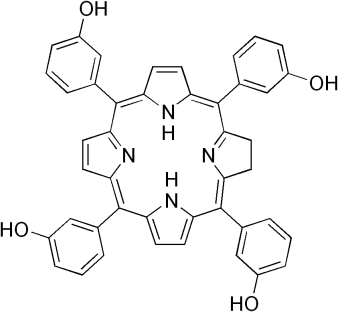
Temoporfin is a molecule with a structure similar to the ring structure found in chlorophyll, the chemical used by plants in photosynthesis. A whole range of different photosensitizers has been developed.
During the entire treatment process the patient is light sensitive, the drug being only gradually leached out of the system by gradual exposure to artificial and daylight. This is clearly a major drawback of the technique. Further problems include the calculation of the correct light dose to all aspects of the tumour and the safety margin of normal tissue around the tumour.
There are dramatic examples of effectiveness of PDT but equally there are examples where it fails spectacularly. The evidence base is not sufficient for PDT to be internationally recognized as a standard treatment alternative in head and neck cancer although in the treatment of some other body sites it can be considered as a recognized alternative. The British NHS has recently stopped paying for mucosal head and neck cancer treatment with temoporfin PDT.