Alcohol
Alcoholic beverages, produced by fermentation of carbohydrates, have been consumed for thousands of years in one form or another. Indeed, the development of agriculture as the foundation of civilisation is thought to owe itself to the cultivation of grains for beer brewing. Over time and locations, attitudes vary from prohibition in twenties America, to binge drinking in modern times in the UK, to continued bans of alcohol for religious reasons. Alcohol is probably the most widely used and abused drug on this planet.
Alcohol - consumption and its journey through the body
Alcohol, that is ethanol (or ethyl alcohol), EtOH, is a very small molecule (see Figure 1) that is readily soluble in water. EtOH and water are completely miscible and EtOH can easily transgress biological membranes, including the blood-brain barrier. The energy content of ethanol is considerable, 7.1 kcal/g (which can add to ‘invisible’ caloric intake).
EtOH is mainly absorbed from the intestines. The effects experienced of EtOH on the body primarily depend on the amount ingested. In addition, the EtOH absorption rate by drinking also depends on the EtOH concentration of the beverage, on the CO2 content of the beverage (EtOH from fizzy drinks is more readily absorbed), on the speed of drinking, on any foods eaten around the time of drinking (composition, amount and timing), gender (because there are different fat/water ratios in male and female bodies), individual EtOH drinking habits and history. Absorption rates are individually extremely variable. There are studies eluding to possible health benefits from moderate alcohol intake (such as a glass of red wine with a meal in a Mediterranean-style diet).
Table 1 summarizes some general trends of EtOH effects as a function of blood alcohol content (BAC).
Table 1: BAC, blood alcohol content, and typical effects
| Blood alcohol content /vol. % | Typical effects |
|---|---|
| 0.02-0.03 | Elevated mood, slight muscle relaxation |
| 0.05-0.06 | Relaxation, increased reaction times, decreased fine coordination |
| 0.08-0.09 | Euphoria; impaired balance, vision, speech, hearing, thought |
| 0.14-0.15 | Severe impairment of physical and mental control |
| 0.20-0.30 | Loss of physical and mental control |
| 0.40-0.50 | Coma, danger of death caused by respiratory depression |
| >0.50 | High risk of death by acute poisoning and respiratory arrest |
More than 90 percent of EtOH is metabolised in the liver (accordingly, continued excessive alcohol consumption primarily damages the liver) in a stepwise mechanism involving a range of enzymes as summarized in Figure 2.
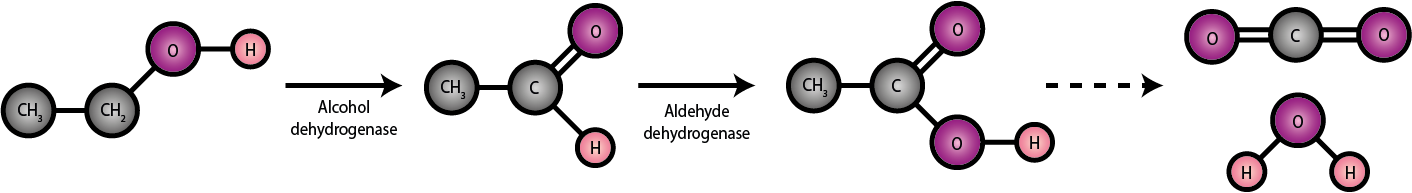
First, EtOH is oxidised to acetaldehyde (a chemically reactive and toxic substance) with the help of the enzyme ADH (alcohol dehydrogenase). This is followed in the next step by further oxidation to acetic acid / acetate, enabled by the enzyme ALDH (aldehyde dehydrogenase). In the final step further oxidation yields water, H2O and carbon dioxide, CO2 which are excreted via urine, sweat and breath.
The enzyme ADH is easily saturated, even at low EtOH blood content, so ADH determines the overall metabolic rate in a healthy person (the ALDH promoted oxidation of acetaldehyde to acetic acid / acetate is not rate-limiting). Any accumulation of aldehyde from excessive EtOH intake is associated with headache, nausea, gastritis and unpleasant hangover effects. In addition, acetaldehyde molecules tend to form stable conglomerates with some nucleic acid molecules (lysine, in particular) and these complexes trigger inflammatory reactions of the liver tissue.
Having to process and to metabolise large quantities of EtOH does lasting harm to the liver by negatively interfering with many of the liver’s metabolic pathways, in particular with its many roles in oxidation and reduction reactions. For example, the central role of liver metabolism in the energy production for the body is impaired: the synthesis of glucose (the body’s ‘energy pack’ sugar molecule) is hindered because pyruvate is prevented from playing its usual role in the production of glucose and instead is used up in the production of excess lactate, with the further consequence that ureate is no longer properly excreted via the kidneys, uric acid accumulates, leading to gout and a condition called lactic acidosis (build-up of lactate in the body, making one ill). Impaired glucose synthesis can lead to dangerous hypoglycaemia (low blood sugar levels) from heavy drinking. The EtOH metabolism also interferes with the normal balanced processing and production of fatty acids by promoting the synthesis of excess fatty acid, leading to a condition called ketoacidosis (build-up of ketones in the body from lowered pH value (higher acidity)). The oxidation rate of lipids is reduced, leading to an accumulation of fat in liver cells, especially triglycerides. Over time, a fatty liver deteriorates via a hepatitis stage to a liver damaged by fibrosis (scarring of connective tissue) and finally cirrhosis (severe and irreversible scarring of the liver tissue) and fatal liver failure. Disruption of liver function by EtOH metabolism further causes electrolyte imbalances in the body (especially Ca2+ and Mg2+) and deficiencies in vitamins, particularly folate, thiamine and vitamin B6.
Elimination rates of EtOH from the body are individually highly variable, with the highest elimination rates occurring for alcoholics during detox:
Alcoholics during detox > healthy binge drinkers immediately after a heavy drinking session or long-term drinkers during treatment with a high-protein diet > heavy long-term drinkers (no liver damage yet) > healthy moderate drinkers >> people with liver dysfunction (cirrhosis), poor diet, malnourishment, or taking some drugs that block ADH (for example cimetidine, an over-the-counter inhibitor of stomach acid production, which is often taken in parallel with EtOH).
Alcohol – how and why it is an addictive drug
Nobody needs any lecturing about the potentially devastating effects of EtOH addiction on individuals, their families and friends, and society overall. And nobody needs reminding of the addictive properties of EtOH. But it may be helpful to consider in a little more detail how the EtOH molecule acts in the brain, in multiple direct and indirect ways: this will illustrate rather well why the story of EtOH abuse is a rather complicated one!
To start with, there is not even a clear cut and simple definition of the meaning of the term ‘alcoholism’ – or better: ‘alcohol dependence’ (and since 2013 as the preferred term ‘alcohol use disorder’).
Several criteria have been collated:
- Developing tolerance (see below)
- Withdrawal symptoms (see below)
- Using larger amounts than intended
- Inability to reduce consumption
- Disproportionate amounts of time dedicated to sourcing alcohol and/or suffering from hang-over
- Increasing social isolation due to alcohol consumption
- Continued excessive use despite knowledge about harm.
There seems to be agreement that if at least three of these criteria are fulfilled over a period of at least twelve months, this is an indication of alcohol dependence. This approach certainly is an indication that the story of EtOH as an addictive recreational drug is indeed complicated, with a high degree of individual variability.
When thinking about the addictive power of nicotine, the underlying mechanisms there seem comparatively straightforward: by and large, the nicotine molecule transgresses the blood-brain barrier, it finds its preferred receptor site to interact with – one that happens to lead to increased release of dopamine (the substance in our brains that is related to pleasurable ‘reward’ experiences and that is in some ways involved in the working of nearly all addictive substances), which explains the addictive power of nicotine.
The small EtOH molecules are also easily able to transgress the blood-brain barrier, and if it wasn’t for the actions of EtOH in the brain, it would not be an addictive drug. Other than with nicotine, there is not one single, or even one main, mechanism how EtOH impacts on brain function. The small EtOH molecules diffuse to several sites in the lipid bilayer of neurons (the communication nodes in the brain) and interfere there with several different neurotransmitter functions and receptors in direct and/or indirect ways (neurotransmitters are messenger molecules, responsible for communication and chains of command amongst the network of neurons in the brain). Figure 3 attempts to give an (simplified) overview of some of the main EtOH interaction mechanisms in the brain.
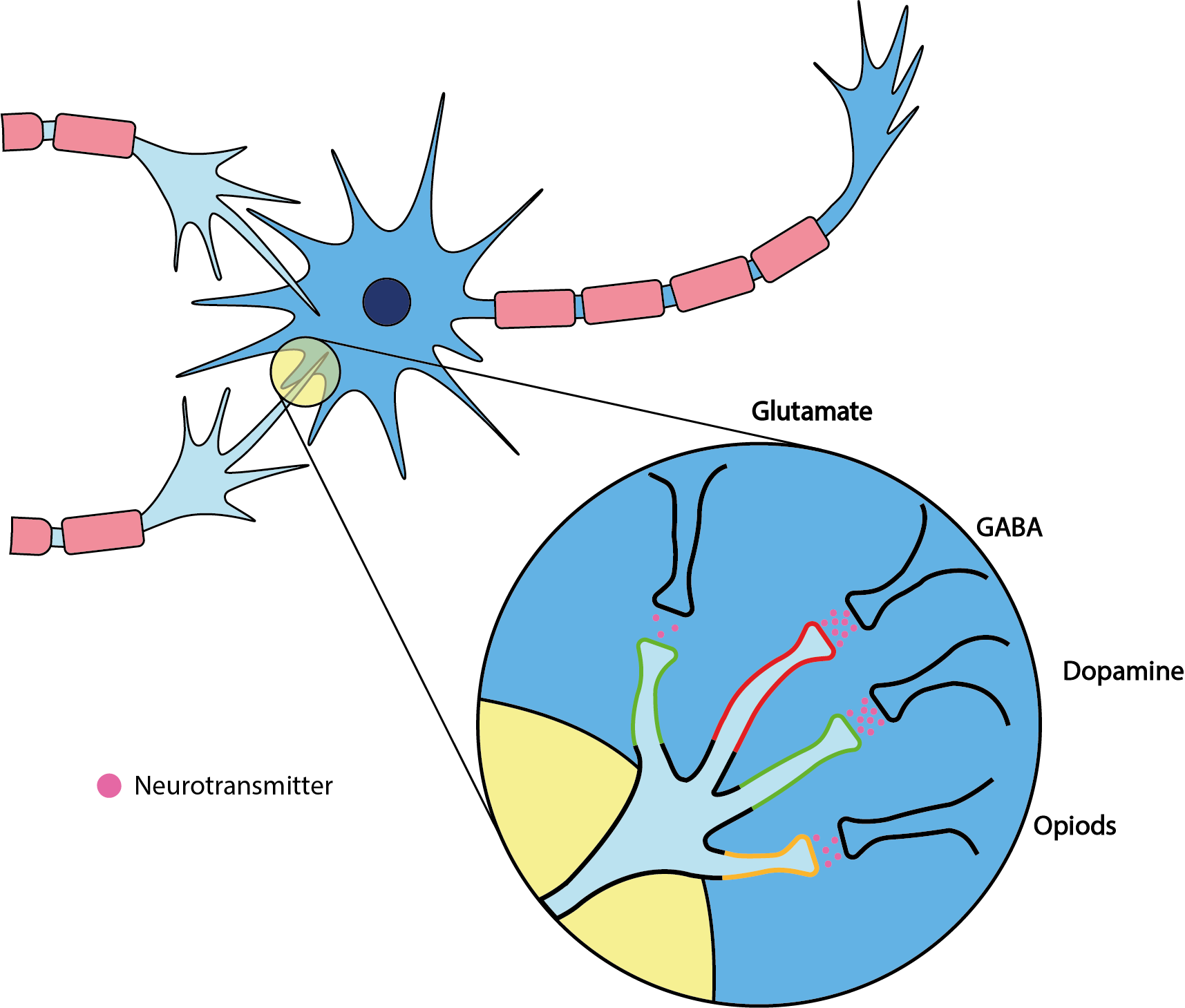
The main interaction sites of EtOH are receptors for neurotransmitters with different properties:
- GABA (gamma-aminobutyric acid) is the main neurotransmitter with inhibitory effects (sedative and anxiolytic); EtOH interfers with the corresponding receptor sites and leads to its downregulation (that is, less activity and less release of GABA).
- Glutamate is the main excitatory (stimulant) neurotransmitter; EtOH interferes with the corresponding receptor sites (NMDA (N-methyl-D-aspartate) receptors) and inhibits the stimulant effects.
- Dopamine is the substance responsible for pleasurable reward sensations; EtOH increases the release of dopamine.
- Opioids are not all synthetic laboratory-made substances, the body / brain has its own opioid synthesis pathways for two types of substances (endorphins and enkephalins) with effects similar to those of synthetic opioids (relief of pain and anxiety); EtOH leads to increased release of these endogenous (made by the body) opioids.
Having a massive impact on neuronal pathways with such different properties explains why different concentrations of EtOH have seemingly contradictory effects as stimulating or as sedative drug: at low concentrations simply the inhibition of inhibitory pathways is the main effect (hence the overall slightly stimulant effect at low EtOH concentrations), whereas with increasing EtOH concentrations the resulting effect is increasingly a sedative effect.
EtOH has profound and multiple effects on the overall brain function, and there is no known antagonist for EtOH (that is, there is no known substance that counteracts all the effects of EtOH). These complicated and multiple different direct and indirect interactions of EtOH with the entire neurotransmitter network also make it plausible why EtOH interferes with numerous medicinal drugs, where, for example, it potentiates the effects of other sedatives (such as barbiturates and benzodiazepines), why complicated and individually different patterns of abuse and dependence develop, and how excessive alcohol consumption over long periods of time not only damages the liver.
An important part of the dependence-forming properties of EtOH is not just related to the involvement of enhanced dopamine release, but the rapid development of tolerance. Becoming tolerant to a drug means that more of it is needed to achieve the same effect as previously with a lower dose. This happens because after repeated exposure neuroadaptation occurs, increasing the number of relevant receptor sites (alcohol inhibits the NMDA / glutamate receptor, so this receptor gets upregulated; the GABA receptors become less sensitive to the effects of EtOH and the enhanced dopamine release becomes less, as well as the opioid receptor responses becoming less) as well as leading to overall changes in absorption, metabolism and excretion. With EtOH, both acute and chronic forms of tolerance develop. Tolerance leads to increased consumption, it maintains and supports dependence and increases the risk of harm. In parallel cross-tolerance to other sedatives develops, carrying increased risks of overdosing some substances. With EtOH also some behavioural tolerance evolves over continued excessive EtOH consumption: to some extent, the body learns how to cope while being intoxicated. In addition, there are several reinforcement mechanisms in place, all pushing toward continued consumption. Some of these are positive reinforcement mechanisms, such as gain of pleasure or being in tune with peer behaviour; other reinforcement mechanisms are the (urgent and strong) desire to avoid withdrawal symptoms, or to relief stress in general.
Suddenly stopping prolonged excessive drinking habits leads to EtOH withdrawal symptoms. These symptoms may start soon after the last drink, within hours, and may persist for several weeks. The withdrawal symptoms include irritability, increased heart rate, headache, sweating, nausea and vomiting, hallucinations, severe anxiety and tremors. Serious EtOH withdrawal symptoms (delirium tremens) are a medical emergency (with a mortality of ca. 5 percent).
From the above description of the multiple interactions of EtOH with multiple neurotransmitter systems, it is straightforward to see that a sudden return to no-EtOH exposure leads to some hard-to-control hyperactivity of numerous neurotransmitter pathways in the brain. The multiple mechanisms in which EtOH interfers with normal brain function also provide opportunities for medication to support medically controlled and supervised detoxification. The most widely used drug to support detoxification is a benzodiazepine derivative, chlordiazepoxide; it reduces some of the withdrawal symptoms (convulsions and hallucinations) and cravings. A different mechanism is exploited by disulfiram: it blocks the ALDH receptor (see Figure 2, above) which leads to acetaldehyde accumulation and related unpleasant vomiting, making drinking EtOH altogether a very unpleasant experience. This is why metronidazole (an anaerobicidal antibiotic drug with a similar molecular structure) must not be mixed with alcohol in any form. A third mechanism to support EtOH detoxification is the use of a drug that efficiently blocks the rewarding, dopamine-related effects from consuming EtOH (naltrexone).
Perhaps some extra-motivation to quit excessive alcohol use when confronted with oral and maxillofacial surgery: there are multiple enhanced risks during and around the time of surgery, leading to higher morbidity and increased risk of post-operative complications as well as higher risks from general anaesthetics during surgery. Many of these risks are related to acute alcohol withdrawal symptoms. Several drugs commonly used post-surgery (antibiotic and antifungal medications) interfere negatively with continued alcohol consumption. Excessive prolonged alcohol consumption is directly toxic to the oral mucosa; this toxicity is likely to be due to exposure to EtOH as well as to its metabolite acetaldehyde (see Figure 2). This mechanism is thought to underpin the role of EtOH in oral tumour promotion because of increased cell regeneration, as well as obviously inhibiting healing of the oral mucosa.
Unless you are faced with emergency surgery, there is usually enough time before elective (planned) surgery to engage in a supported round of pre-surgical detoxification. With the right kind of motivation and support in place, this can be done at home which is more pleasant for you and at least as effective as a much more expensive admission to hospital for essentially the same process.