Saliva and eating
Contents
Composition of saliva
Saliva is a nearly neutral (that is neither alkaline nor acidic) aqueous solution. Like all body fluids it contains a number of electrolytes (that is inorganic ions) such as Na+, K+, Ca2+ and Mg2+ cations, and Cl-, HCO3-, PO43-, I- and F- anions. Saliva is super-concentrated in Ca2+ and PO43- ions and in this way prevents teeth dissolving in this aqueous electrolyte solution. The electrolytes, in particular HCO3-, act as a buffer (that is they balance and maintain the pH value such that there are no large pH fluctuations).
The taste of salt only is noticed if the concentration of Na+ ions in the food is higher than the natural concentration in saliva. Some or all of the electrolytes are also likely to play a role in activating taste buds.
Organic components of saliva include a number of proteins and amino acids. Of the proteins in saliva amylase, statherin and mucins are most relevant for the process of chewing, tasting and swallowing. Other proteins such as lysozyme or lactoferrin are more important for the role of saliva in protecting teeth and oral health in general.
Functional organic components of saliva relevant for eating
Statherin
Statherin is a typical example of an amphiphilic organic / biochemical molecule, a phospholipid with a polar hydrophilic end (hydrophilic means water loving) and a non-polar hydrophobic end (hydrophobic means water hating, sometimes equivalent with fat loving). The vastly different properties of the two ends of such molecules account for their important roles in living creatures as well as in technical systems.
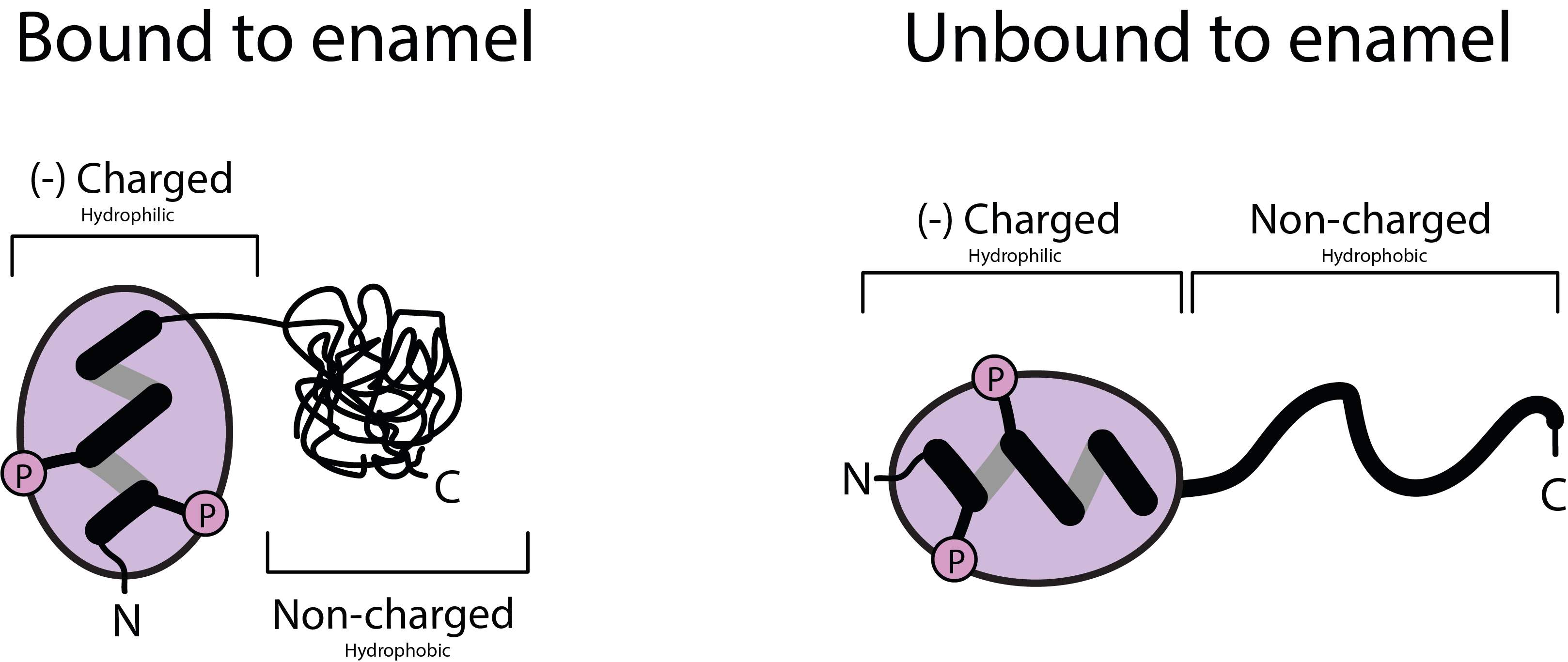
Figure 1 illustrates the overall structure and function of the statherin molecule. The left part of Figure 1 shows the molecule in its folded up conformation, broadly exposing its hydrophilic polar end. In this conformation the polar phosphate end of the molecule attaches itself to the surface (the enamel) of teeth where it helps to protect teeth and to yield a smooth surface of teeth in order to protect tongue and mucosa. This is sometimes termed a “biofilm”. The right part of Figure 1 shows the statherin molecule in its unfolded elongated conformation. In this form it functions mainly as an emulsifier of food, helping to produce a smooth and slippery bolus that is easy to swallow.
Amylase
Amylase is a typical example of a large protein molecule belonging to the class of enzymes. Enzymes are nature’s biochemical versions of a man-made technical catalyst: they help to make a (bio)chemical reaction happen but do not get used up or modified in the process. Like almost all catalysts, enzymes typically depend on the presence of some metal ion(s) in order to function in their role (think of the noble metal catalyst in the exhaust of a car that enables the conversion of toxic gases to less toxic ones).
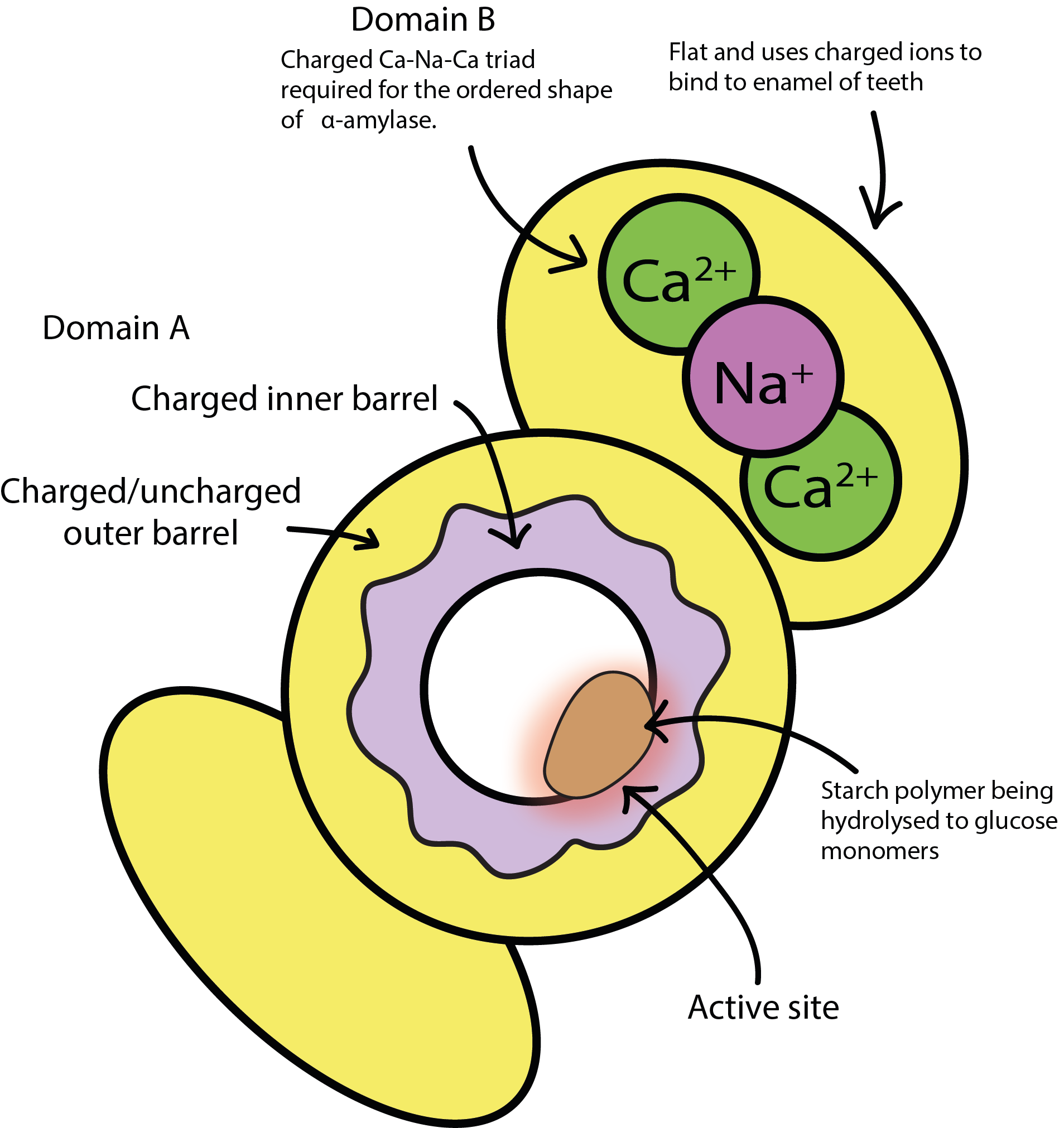
Figure 2 shows a sketch of the overall structure and function of the alpha-amylase molecule. Its function as an enzyme depends on the presence of Ca2+ and Na+ ions. Its role as an enzyme is to enable the breaking down of large carbohydrate molecules in food (such as starch in potatoes) into smaller units while chewing. This helps both with pre-digestion and with the production of a soft and easy to swallow bolus. The related role of alpha-amylase in terms of oral health is to help clearing the mouth from debris after eating, including hard to reach spaces such as between teeth. Alpha-amylase is mostly contained in the on-demand saliva made in the parotid gland while eating.
Figure 2 further highlights an important general principle in all biochemical reactions – form and function need to go hand in hand: only if the size and shape of a starch molecule fits very well with the grooves and overall shape of the alpha-amylase molecule will there be a chance for a successful reaction. The donut-shaped barrel in the centre of the alpha-amylase molecule is its active site and is perfectly set up to accommodate a starch molecule in order to trigger the breakdown of the large starch molecule into smaller (sugar) units (starch is a large polymer molecule made up from many small sugar molecules (glucose monomers) fused together). The underlying actual chemical reaction is a simple acid/base reaction.
Mucins
Mucins are a family of typically very large molecules made up from a protein backbone with ordered and chaotic sections and side-branches consisting of sugar molecules. Two particular mucins are found in saliva: a smaller one (MUC7) as depicted in Figure 3 (top) and a very large one (MUC5B, Figure 3 middle).
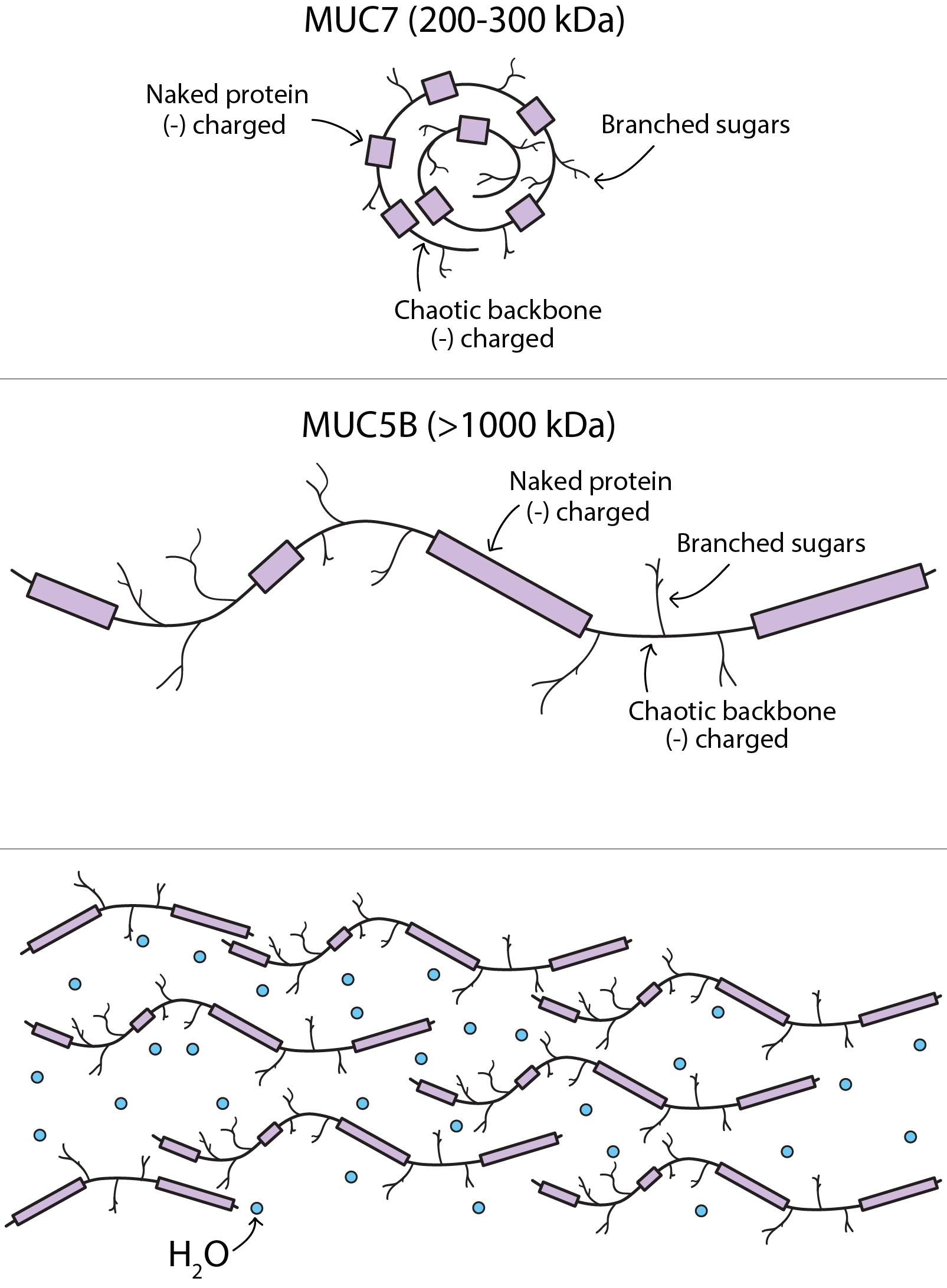
Their role is predominantly to give saliva its viscosity, making it sufficiently sticky to not be easily washed away. In terms of interactions with food and swallowing, especially the elongated very large MUC5B molecules have an important role: because of the ability of the branched sugar side-chains to hold on to water molecules (illustrated schematically in Figure 3 bottom), MUC5B can form very large aggregates which will give particular degrees of slipperiness and viscosity to a bolus and are thus likely to play an important role in the signalling and regulation of the swallowing process – as they render saliva a non-Newtonian fluid (that is a fluid the viscosity of which depends on the shear forces acting on it).
Artificial saliva
Given the truly important role of saliva in enabling eating, chewing and swallowing, it comes as no surprise that quite a selection of so-called artificial salivas are commercially available for people suffering from dry mouth. It probably comes as no surprise that there really is no artificial replacement available that would come anywhere near the amazing properties of natural saliva. It is difficult to mimic the properties of saliva, given the complicated feedback loops and production modes, depending on demand or rest that govern the properties of natural saliva. But it does not hurt to try if any of these artificial preparations help to relieve symptoms of dry mouth and/or help with eating.
Commercial artificial saliva preparations come in quite a range of compositions. Some essentially just mimic the inorganic electrolyte composition of ‘whole mouth’ saliva (a mixture of on-demand and at-rest saliva). Some products are more focussed on the organic components of saliva with either an emphasis on (some form of) mucin, or on proteins with a major role in maintaining oral health. The available preparations include sprays, lozenges or gels. It makes sense to avoid products that are acidic in order to protect your teeth and to prefer products that contain F- ions to enhance the protection of teeth.
Many patients report that the various different kinds of artificial saliva merely provide some minor improvement of mouth feeling, some fresh feeling often predominantly from the flavouring added to the preparations. Some people report that in particular gel preparations are helpful with prolonging uninterrupted periods of sleep. With regard to ease of eating and swallowing some alternative coping strategies may individually work better.