Lubricating food
Contents
Eating difficulties caused by severe forms of dry mouth are a common cause of misery for maxillofacial patients, especially for people who underwent radiotherapy treatment in the head and neck region. Unfortunately, these difficulties often persist for a considerable period of time, and in some cases become a permanent problem. Artificial saliva usually does not solve eating problems of this kind. Using large amounts of water to literally wash food down helps but only goes so far - having to forcefully wash down food certainly does not create a pleasant eating experience.
For some people it may be sufficient to mainly eat moist foods and use lots of sauce(s) with all meals, combined with drinking lots of water. For others slightly more engineering is needed to make meals more manageable and more enjoyable. Depending on the exact nature of some swallowing problems, it may be better to keep the texture of a lubricant completely smooth and fairly thick, while others will prefer a slightly thinner lubricant with soft but textured food, and so on.
We use the term engineering here because the mechanisms of swallowing and problems with swallowing when some components in the process fail can explain which textures and lubricant consistencies are likely to work best. In addition, the mechanisms of the following two, fairly general food lubrication tricks are fully explained by the underlying chemical and physical principles and are based on the current understanding of how saliva enables chewing, eating and swallowing.
We will have a look at the effects of a natural emulsifier and a gelling agent and how this can explain their roles as lubricants. Please do remember – lubricating your food is not the same as diluting it with plenty of liquid but rather aims to achieve an overall smooth and sufficiently slippery bolus that will be easy to swallow, similar to the consistency of a food bolus produced with the help of natural saliva.
Emulsifying agents
The following is actually a story of the power of ‘citizen science’ and communication in making sense of what people report repeatedly and independently of each other. Time and again, people afflicted by dry mouth problems report that (non-sugary) proper old-fashioned custard is a great lubrication agent and helps with eating in general, savoury as well as sweet foods. Hearing all these reports one is initially tempted to think that this is simply due to the fact that custard is such a well-established comfort food and comes with a lovely non-aggressive taste as well as a vanilla flavour that we can notice by its smell rather than its taste. While that is surely part of its success it is only part of the story.
There is the odd report where people mention that they find off-the-shelve commercial salad cream useful as a lubricant for everything, including chocolate cake. One is initially tempted to ascribe such reports to the ingenuity and inventiveness of people in overcoming difficulties – if taste is disturbed anyway, why not use salad cream for lubricating everything, if it works.
Eventually, the detective instincts kick in and the question cannot be ignored any longer: What actually do custard and commercial salad cream have in common, if anything? Obviously they do have in common that both lubricate a range of foods reasonably well. And they have in common that both contain an emulsifier. Proper custard is made with egg yolks, egg yolks in turn contain lecithin, a natural emulsifier. Commercial salad cream needs to be stabilised for a prolonged shelve life and to prevent it from splitting. That is achieved by adding an emulsifier to the preparation, either lecithin (in this case more likely derived from soy beans rather than from egg yolk) or some other powerful thickener such as xanthan gum.
Statherin, one of the proteins in natural saliva, relevant for the role of saliva in enabling eating, chewing and swallowing, actually is another example of a naturally occurring emulsifier. Figure 1 compares the overall structures of these two different phospholipid molecules, statherin and lecithin. Phospholipid is the general name for molecules with a polar (phosphate group, attracting water and other polar molecules) end and a non-polar end opposite that tends to attract other non-polar molecules (such as a fat).
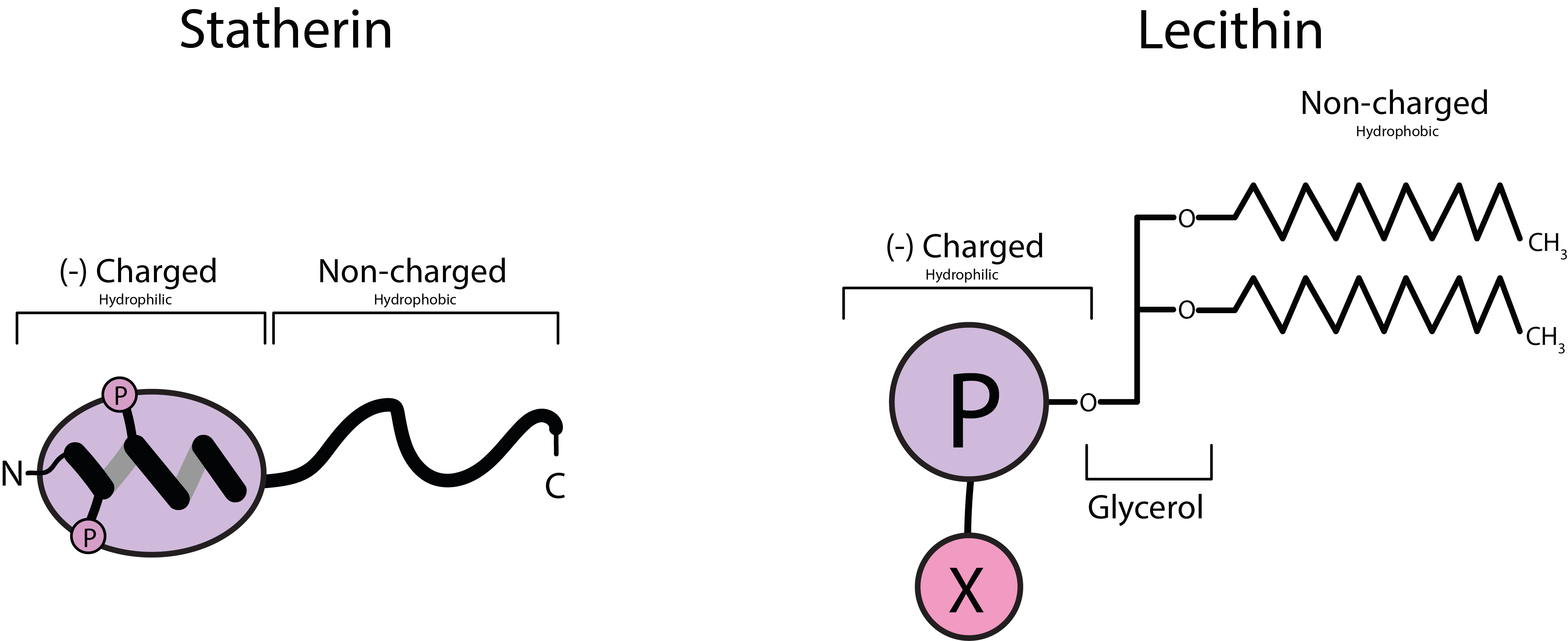
The left part of Figure 1 shows a sketch of the overall structure of a statherin molecule, the right part of Figure 1 depicts the overall structure of a lecithin molecule. Even if lecithin overall is a much larger molecule, the two compounds have identical functional units. These units enable both compounds to ‘wrap up’ and coat fatty (non-polar, hydrophobic) food particles in a watery environment to produce homogeneous emulsions. This versatile general function of phospholipids as emulsifiers is illustrated in Figure 2.
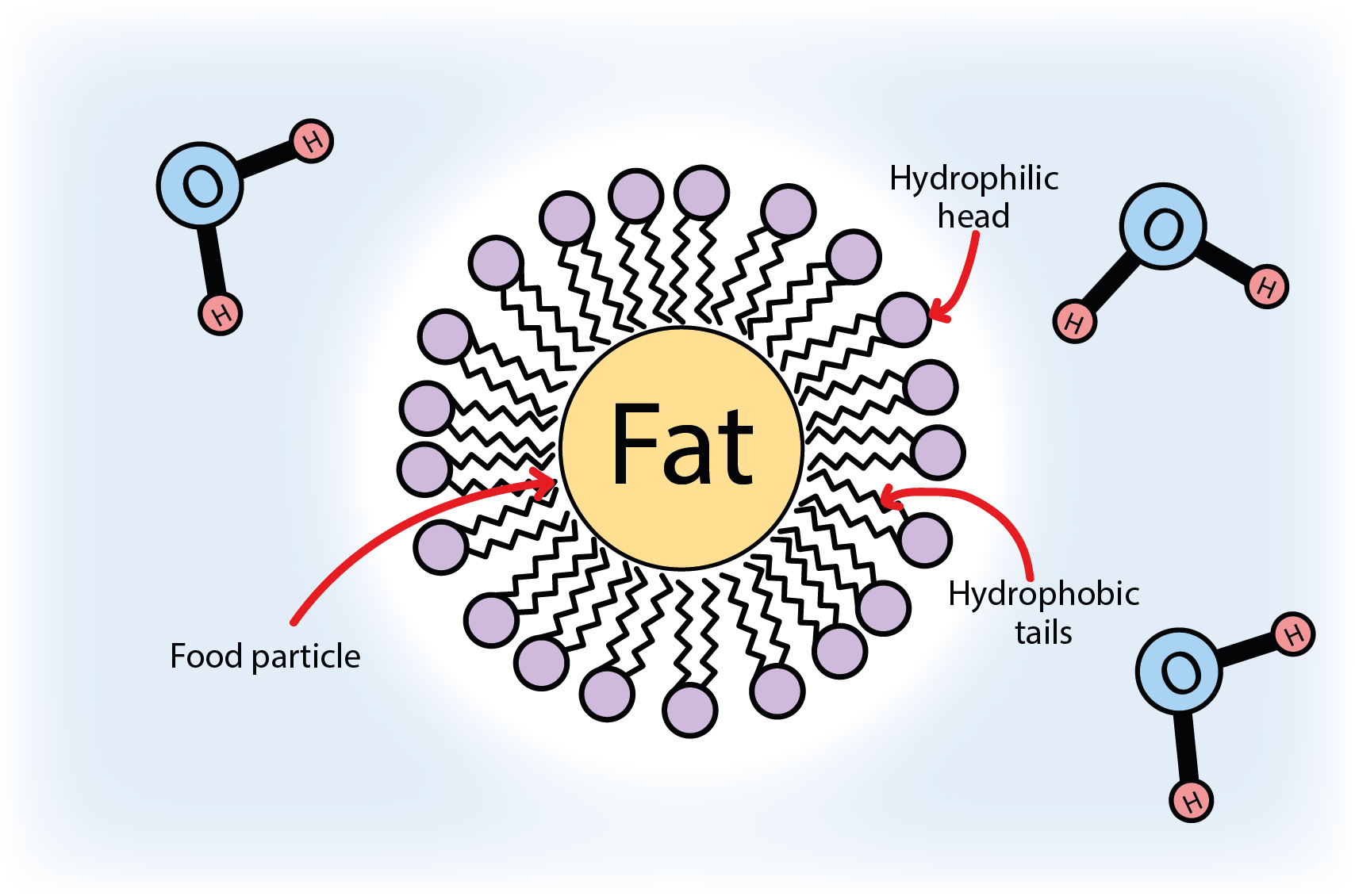
This general functionality of phospholipids as emulsifiers explains well how lecithin (in egg yolks in custard…) can replace to some extent the lubricating function of one of the components of saliva relevant for eating, statherin, and thus can be instrumental in overcoming dry-mouth related eating problems.
In purely practical terms we can see that egg yolks will be a versatile (and nutritious) natural ‘additive’ to help with lubricating many foods. Egg yolks will give a silky and smooth consistency to many foods. For example, just add an egg yolk to vegetable soup before serving but do not boil the liquid anymore. The emulsifying properties of egg yolks are also responsible for the smooth and silky consistency of many home-made ice creams and other preparations such as custards or flans (baked custards).
Gelling agents
Not only are gelatin-based, melt-in-the-mouth jellies easy to eat in many circumstances, there are also reports that softly set, wobbly jellies act as good lubricating agents for a range of other foods. How and why does that work?
One of the proteins in saliva, MUC5B, is a rather large elongated molecule (see Figure 3, top). Due to its many polar side branches it can form very large aggregates by accommodating considerable amounts of water molecules (illustrated in Figure 3, middle). This is called gelling and is (one of) the mechanism that makes saliva a rather sticky (non-Newtonian) liquid. Sticky saliva is good at wetting all kinds of dry foods and contributes to the formation and transportation of a homogeneous and soft, easily swallowed bolus.
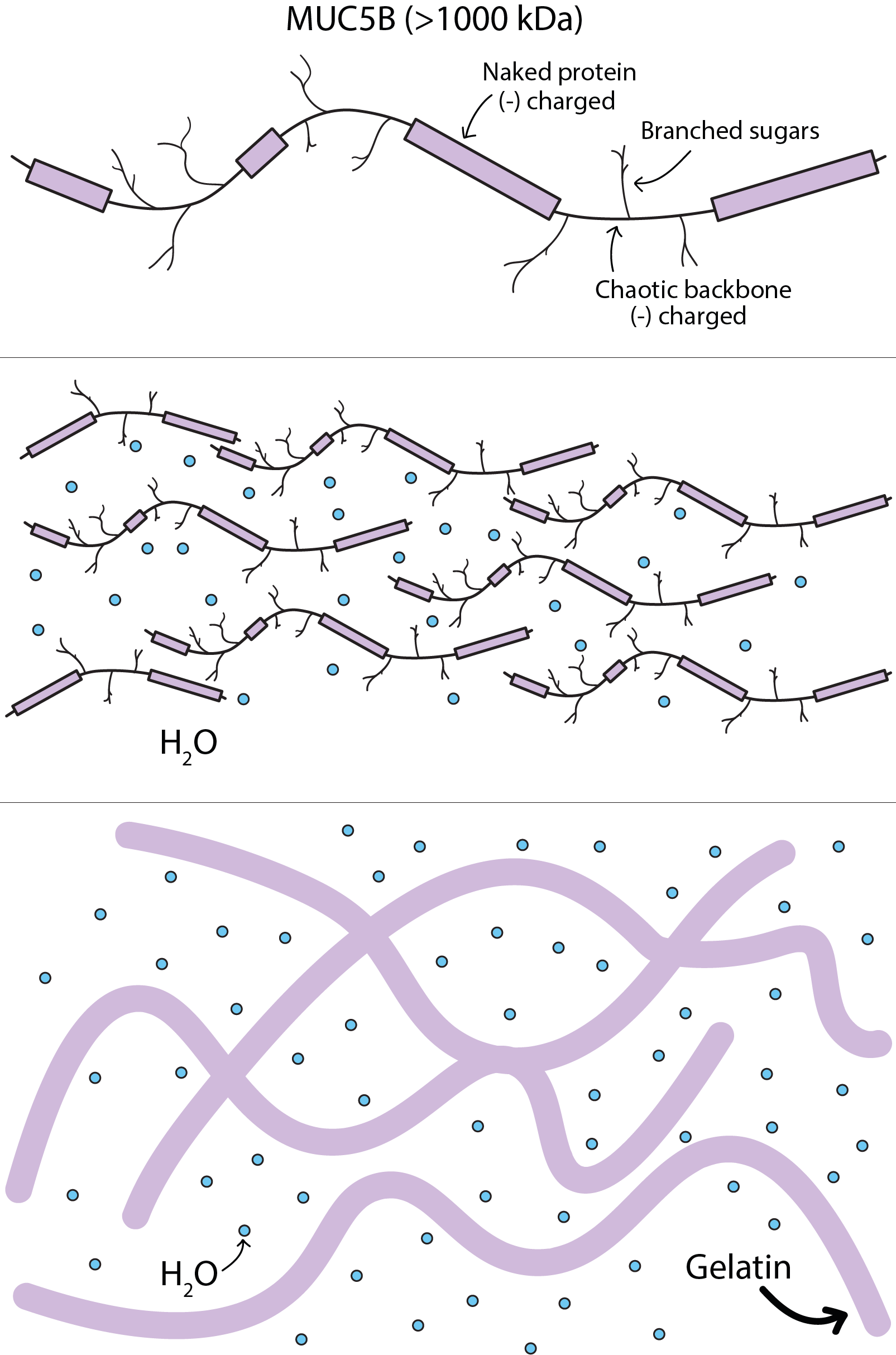
With too little saliva, or in the absence of natural saliva, water alone cannot make up for this wetting and lubricating role of the MUC5B protein when eating: water lacks this crucial not-quite-liquid texture for this particular task. However, pairing food with another jelly, very softly set and especially when made with ordinary gelatin, can partially compensate for this. The bottom part of Figure 3 illustrates the overall structure of gelatin: large, elongated protein molecules with polar sidechains which are able to accommodate large quantities of water molecules, altogether quite similar to the global structure and gelling mechanism observed in MUC5B. When soaking gelling agents such as gelatin in water they will take up volumes of water multiple times their own volume (see Figure 4).

A few general comments about lubricating foods
For most people with eating problems due to dry mouth the best way forward depends on the overall combination of swallowing troubles. The eating experience will usually benefit from a bit of exploration of the most suitable texture and temperature of foods and corresponding recipes. This experimentation is best seen as an ongoing adventure as most dry mouth and other other swallowing problems tend to change over time.
Apart from the two general food lubrication options (emulsifying and gelling agents) that we briefly discussed above, the best approach certainly depends strongly on personal taste and preferences, not just personal patterns of troubles. Potential lubricating agents range all the way from water to pure fat such as molten butter and everything inbetween.
The printable grid (see above) lists a few columns for food categories that may need lubrication. It may be useful to find out (and remember) good combinations of foods and lubricants – and those that do not work well. Do work your way through the grid, including some combinations that may not look particularly convincing at a first glance, perhaps that turns out exactly the combination that works well for you for the time being. Even better, please do share your food lubrication experiences: together we may discover more useful tricks and ideas about improving the eating experience with dry mouth.