Bisphosphonates
Bisphosphonates belong to a class of drugs called antiresorptive agents. These agents interfere with regular bone remodelling by hindering the bone resorption step in this process. Bisphosphonates inhibit the osteoclasts (the cells driving this part of the process) in various different ways. Bisphosphonates are widely used in the treatment of osteoporosis and metastatic bone disease and other bone malignancies, such as multiple myeloma.
Bisphosphonates are relevant in a maxillofacial context because of their adverse effects. These agents are nowadays the most common cause of medication-related osteonecrosis of the jaws and often do require surgical intervention, including major reconstructive surgery.
Figure 1 shows the general chemical structure of a bisphosphonate. Two phosphonate groups (PO32-) and two further groups R1, R2 are bonded to a central carbon atom.
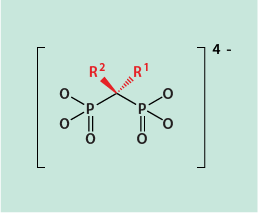
There is a range of different bisphosphonates currently being prescribed (see below). In six out of eight of these, one of the R groups is a hydroxyl (OH) group. The hydroxyl group maximises bone affinity of the drug molecules by easing absorption into the calcium phosphate (hydroxyapatite) bone matrix. The other R group is the main determinant of the potency of a particular bisphosphonate agent (see below).
Figure 2 gives an overview of three generations of bisphosphonates. The first medical use of a bisphosphonate in the 1960s was the prescription of etidronate for the treatment of Paget’s disease, alongside numerous other technical uses of this general group of chemicals. The second and third generation bisphosphonates all contain a terminal amine (NR2) group in one of the R groups. In the third generation bisphosphonates, the amine group is incorporated in a ring structure. Across the range, all bisphosphonates share a high affinity to bond to bone mineral for long periods of time (up to 15 years have been reported). Given their association with increased risk to develop osteonecrosis (MRONJ) following invasive dentoalveolar surgery combined with the lasting presence of these agents in bone, a short interruption of the treatment (‘drug holidays’) to allow for oral surgical interventions makes no sense. The situation is slightly different for other antiresorptive agents, such as denusomab.
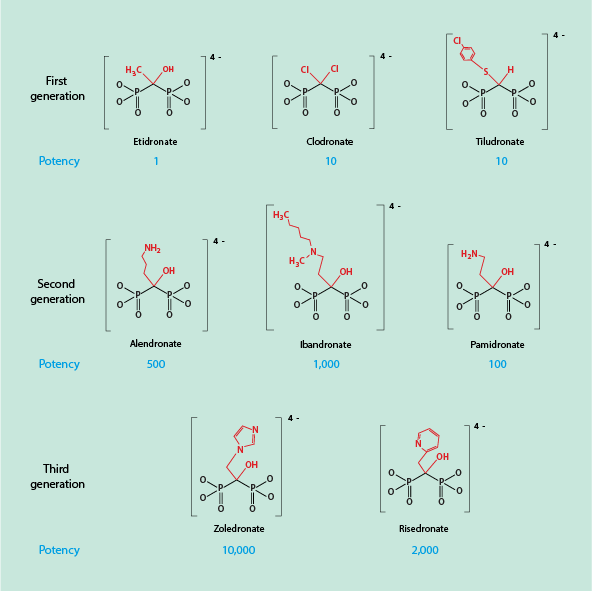
Figure 2 also lists the relative potency of the different bisphosphonates, covering a wide range from a potency of 1 (etidronate) all the way to a potency of 10,000 (zolendronate). This difference in potency is far too large to be explained simply by differences in affinity to the bone surface. The term ‘potency’ with respect to bisphosphonates relates to their inhibitory potency for bone resorption. The numerical values for the relative potencies are derived from measuring the dose of a particular drug that is required to achieve a 50 % reduction of bone resorption. This is called an IC50 value, the half-maximum inhibitory concentration. The higher that required dose, the lower the resulting potency. Osteoclast inhibition cannot be determined directly, but IC50 values for a range of bisphosphonates have been derived from in vivo and in vitro observations. In vivo data are derived from the measurement of biomarkers for bone resorption in blood and urine samples and correlate well with in vitro obtained data.
Bisphosphonates can be administered either intravenously or orally (in tablet form). In terms of actual potency in clinical applications of bisphosphonates, the drug delivery method has an impact too. When administered intravenously, the actual potency is higher as bisphosphonates have a much higher bioavailability in this delivery mode than when administered orally. For example, it was found that alendronate has an average oral bioavailability of less than one percent. A large difference in bioavailability is due to the poor absorption of bisphosphonates through the gastrointestinal tract.
The large difference in potency between 1st generation bisphosphonates and nitrogen-containing bisphosphonates (2nd and 3rd generation) has been (and continues to be) the subject of active research. All bisphosphonates share a high affinity of binding to the bone surface, presumably enhanced by the presence of one R group in the molecule being an OH group. In addition, there must be further mechanisms at work, in terms of specific biochemical targets and interactions.
1st generation bisphosphonates tend to inhibit osteoclast activity by interference with intracellular ATP (adenosine-triphosphate) metabolism. The nitrogen-containing (and far more potent) bisphosphonates work by a different mechanism. They inhibit an enzyme (farnesyl pyrophosphate synthase, FPPS). This enzyme is crucial in the biosynthesis of a number of proteins which, in turn, are essential for the production of biomolecules that are required for the function of osteoclasts (and other cells), such as lipids. This blockage has a profound impact on the function of osteoclasts and is in line with the high potency of the nitrogen-containing bisphosphonates.This mode of action could be seen as a poisoning of the osteoclasts. The current understanding is that the FPPS interaction mechanism is the main mode of action of these nitrogen-containing bisphosphonates but additional potential biochemical targets in the bone metabolism are being investigated. The high affinity of all bisphosphonates to bone is further being discussed as a potential vehicle to deliver other drugs specifically to bone tissue.
3rd generation bisphosphonates have been compared with an alternative antiresorptive agent, denusomab. Whilst treatment with all antiresorptive agents carries the risk to develop osteonecrosis of the jaws (ONJ), there are distinct differences between these two classes of antiresorptive agents. When treatment is discontinued, the effects of denusomab are more rapidly reversed (within a few months) than for bisphosphonates (where persistence of more than 10 years has been observed). The concept of ‘drug holidays’ for denusomab to reduce the risk of developing osteonecrosis after dentoalveolar procedures (such as tooth extractions), is potentially useful but is not a realistic option for bisphosphonate treatment schemes.
Table 1 summarises some of the key properties of denusomab and bisphosphonates.
Table 1 Comparison of denusomab and bisphosphonates
| Bisphosphonates * | Denusomab | |
|---|---|---|
| Category | chemical agent | biological agent (monoclonal antibody) |
| Target; general | high affinity for hydroxyl apatite (bone mineral); inhibition of enzyme (FPPS) | high affinity and selectivity for RANKL receptor |
| Target; cellular | mature osteoclasts; possibly some effects on osteocytes | precursors of osteoclasts and mature osteoclasts |
| Mechanism of action | inhibition of osteoclast resorptive function(s) | prevents formation, function and survival of osteoclasts; depletion of osteoclasts |
| Distribution | bone mineral surface | circulating (blood and extracellular fluid) |
| Onset of action | fast (if given intravenously); slow (if given orally) | fast |
| Offset and reversibility of action | slow; reversibility unclear (depending on type of drug and duration of treatment) | (relatively) rapid; fully reversible |
* the range of bisphosphonates in clinical use cover a range of properties (see above)