Platelet-rich plasma
Plasma, the liquid component of blood, and the multiple crucial roles of platelets (small cellular fragments derived from specialised cells in bone marrow) in stopping bleeding, and initiating and supporting healing are described on our pages about bleeding.
The term platelet-rich plasma (sometimes called platelet concentrate) refers to a process where somebody donates a small amount of their own blood, approximately 10 to 20 ml. The plasma is separated from the other, solid blood components in a small centrifuge and thus is enriched in platelets relative to the full blood. The platelet-rich plasma is then applied to a surgical site, for example a bone graft from the pelvis to cover a defect in the mandible. It is thought that the unique properties and various roles of platelets in wound healing may lead to earlier vascularisation and integration of the bone graft at the recipient site, if platelets are present at an enhanced concentration (Figure 1).
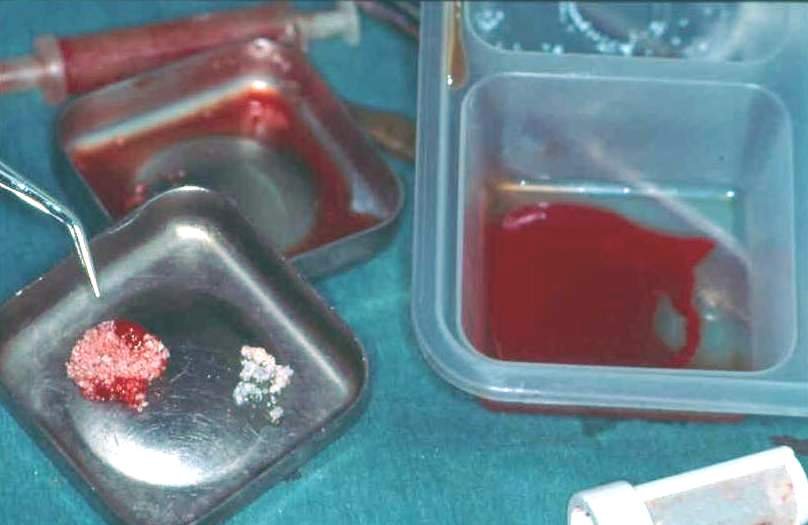
Helped by the ease with which autologous platelet-rich plasma can be obtained and applied, there is an approximately 25 year history of its use in various treatments of surgical wounds and injuries. Platelet-rich plasma is a cocktail of growth factors, cytokines and chemokines (messenger molecules), and some platelet aggregates. Most of the theories and hypotheses about the working mechanisms of platelet-rich plasma in wound healing of hard and soft tissues (including nerve repair) are derived from laboratory experiments and may not necessarily translate smoothly to the clinical situation. Some of the suggested mechanisms are controversially debated. A number of studies carried out on animal models, mostly rodents, demonstrate some capacity and potential for the use of platelet-rich plasma to improve wound healing but the multifactorial situation and the multiple known roles of platelets have so far not been fully elucidated. That is true for these animal models, and also for the use on humans.
Similar to the studies of other potential adjuvant therapies, such as photobiomodulation, there is no scarcity of publications. But because of the commonly poor design and quality of most of the studies, there is no conclusive evidence about the efficacy of the clinical use of platelet-rich plasma. The debate about merits, or lack of merits, of platelet-rich plasma in surgery continues, fuelled by poor data.
Clinical use of platelet-rich plasma has been reported in orthopaedic surgery (especially in the repair of tendon injuries), in the treatment of periodontal disease and related gingival surgery (with no convincing evidence from multiple studies), in the placement of dental implants (again, only conflicting evidence exists), there is some weak evidence that platelet-rich plasma may improve the remodelling and healing of bone grafts and reduce the necrosis of local flaps in maxillofacial surgery, no statistically significant benefit of platelet-rich plasma on the healing of chronic wounds (such as diabetic ulcers) has been demonstrated from a large number of studies, the benefits of platelet-rich plasma in the treatment of neuropathic pain are controversial, at best.
Some have raised concerns that platelet-rich plasma may promote infections and may even play a role in sepsis, given the dysfunction of platelets associated with sepsis. Combining information from a diverse range of studies looking into this aspect (for many different conditions and surgical interventions) actually seems to point toward antibacterial activity of platelet-rich plasma, as had been previously described in in vitro laboratory experiments.