LILT (low intensity light therapy), photobiomodulation
Low intensity light therapy, LILT, also known as low intensity laser therapy, LLLT, has been rebranded as photobiomodulation. The renaming aimed at finding a more precise and less misleading term (for example, light emitting diodes (LEDs) are increasingly used instead of lasers, and there is lack of definition of the meaning of the term ‘low level’). Photobiomodulation describes a variety of phototherapy that is designed to stimulate wound healing and tissue regeneration, amongst numerous other suggested applications.
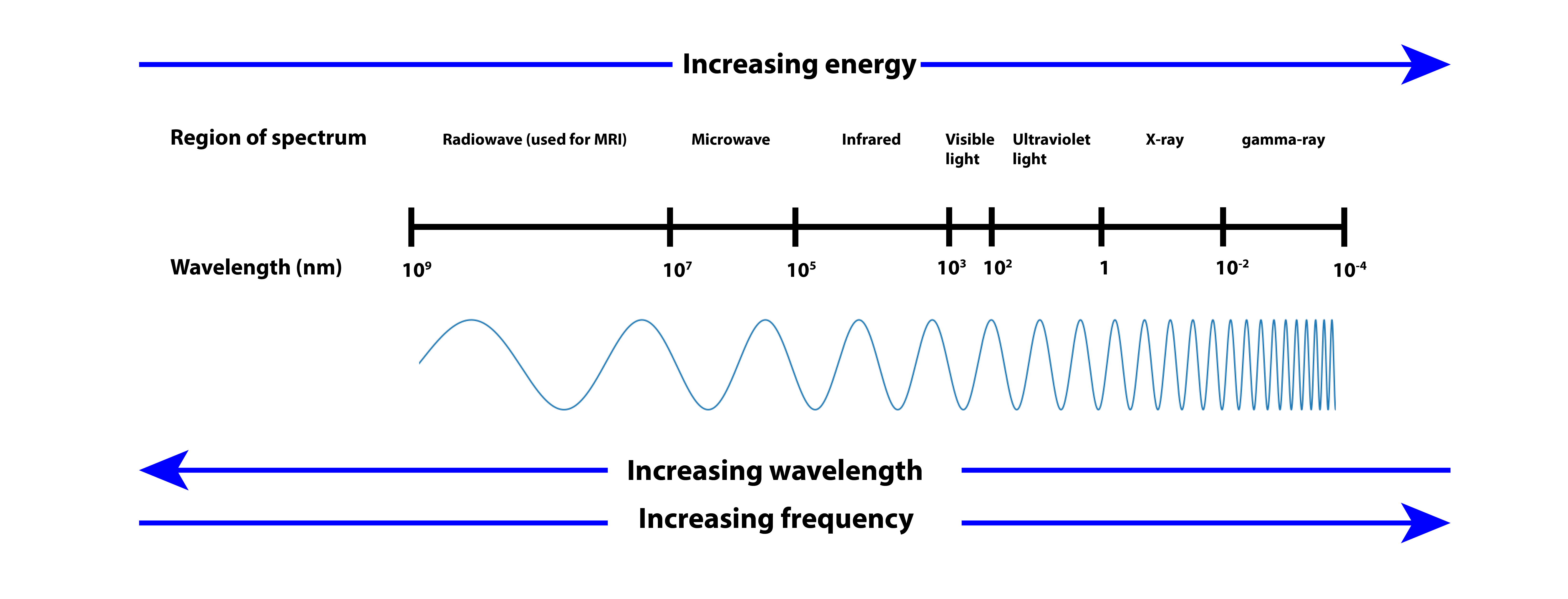
The ideas supporting photobiomodulation relate to the known biological effects of photons (light) of distinct wavelengths on living matter (Figure 1). For example, visible light is important in regulating the circadian rhythm and sleep / alertness patterns; a specific range of ultraviolet radiation (in sunshine) is necessary for the biosynthesis of vitamin D (hence known as the sunshine vitamin) but ultraviolet radiation is also responsible for the ageing of skin and the development of skin malignancies. Photobiomodulation employs radiation in the visible red light to near-infrared range (Figure 1), photons with such wavelengths can be produced by lasers or light emitting diodes, LEDs. Radiation in this wavelength region has been shown to penetrate skin and other tissues.
In order for irradiation to have any physiological effects, it is necessary that a particular wavelength of irradiation is absorbed by some component / molecules in cells. For the wavelengths used in photobiomodulation, it is thought that the responding units are located in the cellular mitochondria (subunits in most cells, involved in energy production and release, via respiration). Specifically, it is thought that the copper ions in a large protein complex (cytochrome c oxidase; a vital part of the processing of oxygen molecules at the cell membrane level) are the chromophores responsive at these wavelengths. Accordingly, it should be expected that cells with high levels of mitochondria and related metabolic activities should be responsive to photobiomoduation (skin cells have low levels of mitochondria). It is not entirely clear how photobiomodulation appears to affect mainly damaged cells and tissues; some hypotheses about this await confirmation (or rejection).
There is a lack of systematic studies in the literature, the exact nature of the causes of experimentally observed increased cell proliferation remains unclear. It is not known if the effects are photothermal, photochemical or photomechanical in nature. Whilst some rodent-model studies demonstrate improvements in the healing of surgical wounds, the effects are not reproduced on pig skin (much more similar to human skin). Similarly, beneficial effects on superficial wound healing in humans reported in small-scale observational studies were not confirmed in larger studies.
Roughly in line with the predictions of the assumed mitochondrial activation mechanisms at work (increasing the release of growth factors, for example), in the laboratory low doses of photobiomodulation irradiation have been shown to enhance the proliferation (growth rate) of endothelial cells, lymphocytes, fibroblasts and keratinocytes. Leading to enhanced vascularisation and angiogenesis as well as increasing collagen production, one might thus expect that photobiomodulation could have a beneficial effect on the healing of acute or chronic wounds. It should also be kept in mind that increasing angiogenesis is not at all desirable in the context of malignant growth, and questions as to the safety of photobiomodulation in those circumstances have been raised repeatedly. A very recent study finds no evidence that photobiomodulation has effects on overall and disease-free survival times, and time to local recurrences in patients with head and neck malignancies treated with radio- or chemoradiotherapy.
This is a relevant point because photobiomodulation has been reported in attempts to prevent and/or treat oral mucositis and mucosal necrosis occurring during and after radiotherapy treatment applied to the head and neck region. The results of the mostly small observational studies are inconclusive and at best there is weak evidence for slightly faster healing. Photobiomodulation has been suggested as an adjuvant therapy for a number of maxillofacial conditions, including various neuropathic pain syndromes, oral lichen planus, periodontal disease and postoperative management following third molar surgery. In all of these cases there is no robust evidence for the efficacy of photobiomodulation treatments provided by mostly poor-quality studies.