Vascular abnormalities
Contents
Below a brief description of the normal development of the vascular system is given; followed by a short discussion of the current thinking about the developmental failures that lead to vascular abnormalities. It may come as a surprise that relatively little is understood about the causes of the most common, sporadic abnormalities.
Development of vasculature
An endothelium-lined complex vascular system is a relatively recent evolutionary trait and only mammals have such a circulatory system. From the point of view of tissue maintenance, delivery of nutrients to tissues as well as removal of debris from tissues, it is a very attractive transport system. It permits high flow rates (laminar flow) and can reach all tissues at all times, flexibly and demand-driven.
The endothelium itself is increasingly recognised as an organ in its own right, rather than just providing the lining of vessels. The metabolism of endothelial cells is increasingly well understood, as is their role in a large number of conditions and diseases, as well as in health. Given that the vascular system is engaged with every part of the body, it is probably not surprising that malfunctions of the endothelium lead to vascular malfunctions, over- as well as under-activity, and are involved with conditions including cardiac and pulmonary pathologies, inflammatory disorders, eye diseases, diabetes and atherosclerosis, and many more.
In the development of an embryo, the vascular system is one of the first units to form. It is an orchestrated process that follows several developmental steps (Figure 1).
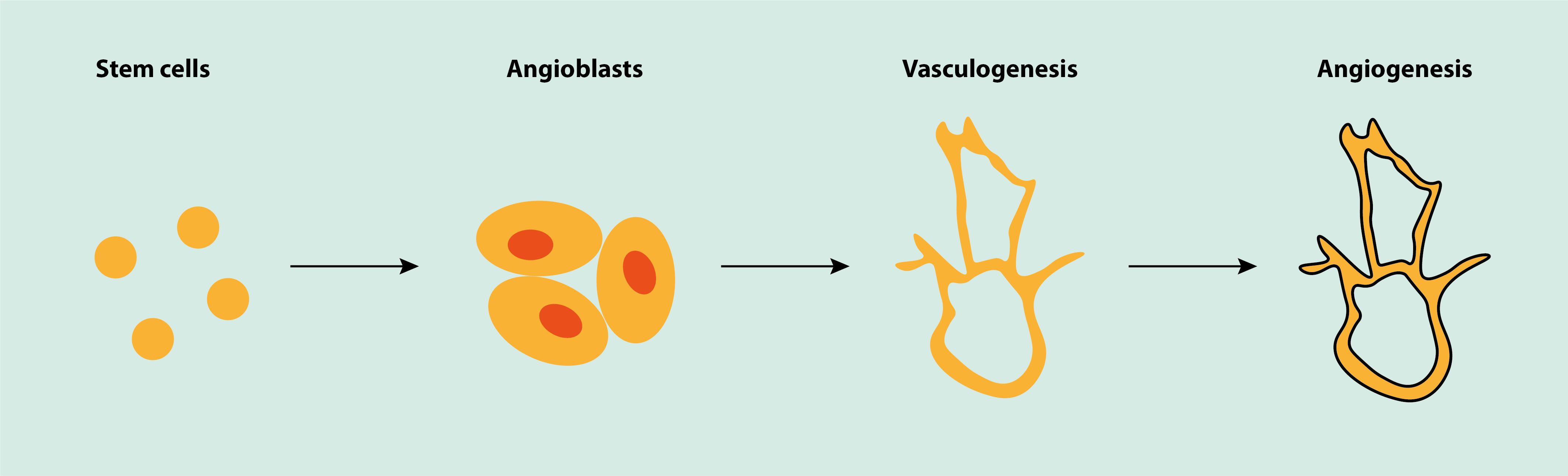
The initial step in the development is the differentiation of precursor cells (angioblasts). Angioblasts are undifferentiated cells which initially develop into endothelial cells. The endothelial cells form an initial, primitive basic vascular network (vasculogenesis). In the following steps, this initial network grows and develops into a fully differentiated vascular system (angiogenesis). This happens by two mechanisms, splitting of an initial vessel (intussusception), and/or angiogenesis where new vessels sprout. The development of the vascular system also entails the simultaneous growth of smooth muscle tissue that envelops the endothelium to form a fully functional vessel.
Once fully developed, the exact functions and metabolism of endothelium and angiogenesis are different in different tissues. Vascular renewal and growth is different in the embryo (where it starts from scratch) to the mechanisms in an adult (where it happens on the back of an existing vascular system). In an adult, the main mechanism for the growth of new vessels is by angiogenesis. This is true for physiological (for example, after injury) and for pathological processes (for example, angiogenesis driven by a malignancy).
Pathogenesis of haemangioma
It has been said that haemangioma is the most common benign childhood tumour and the least understood. However, more experimental evidence is emerging and some, in part contradictory, hypotheses have been formulated. It may turn out later that these hypotheses are simply different aspects of the overall picture. No specific inherited genetic mutations have been identified in conjunction with the most common forms of haemangioma.
It is undisputed that the proliferation phase of haemangioma is characterised by the presence of hyperplastic (large numbers) endothelial cells, giving rise to growth and incomplete and defunct blood vessel architecture. Some researchers think that this is caused by an intrinsic defect of the endothelial cells (which in haemangioma have been found to derive from a single precursor cell and to be clones of each other). Others think that extrinsic local environment / tissue effects are at the core of the malformation, and that the proliferation of endothelial cells is a response to such external factors. The main such factor cited is hypoxia (insufficient oxygen supply) as a trigger. The finding of clonal cells, however, would rule out extrinsic factors as the sole cause.
Another experimental finding may provide the link between the two hypotheses by identifying a unique pattern of biochemical marker molecules, expressed by placenta tissue and haemangioma, in particular a glucose transporter protein called GLUT1. This protein is expressed in most foetal endothelial cells, but in later development is lost in nearly all tissues, except some microvessels in the placenta and the brain. In fact, looking for GLUT1 in haemangioma tissue has become the standard pathology test to differentiate between haemangioma and other vascular malformations.
If the precursor cells are derived from placental tissue, then replicate by cloning, it will take specific local environmental factors (such as acidity, hypoxia, messenger molecules (cytokines), presence or absence and/or imbalance of growth factors and their inhibitors, possibly including some genetic pre-disposition, and so on) for haemangioma tissue with placental biomarker characteristics to develop in skin (for example). This concept combines most of the current evidence into a feasible combined scenario but requires much more work to be verified (or replaced by some alternative theories).
Many of the growth factors known to be involved in vasculogenesis and angiogenesis are found at increased levels in the proliferation phase of haemangioma, in particular the vascular endothelial growth factor (VEGF). These concentrations decrease with involution of the lesion, when instead levels of biomarkers for programmed cell death, apoptosis increase. The serendipitous discovery of propranolol as a medicinal agent to treat haemangioma fits in with this observation: propranolol is thought to enhance endothelial apoptosis events and may thus simply provide a shortcut to the involution phase of haemangioma. However, it is currently not known what exactly triggers and drives the transition from proliferation to involution of haemangioma. There are more questions that need answers: i) why are females significantly more often afflicted than males, approximately in a ratio 2 : 1; ii) why is the head and neck region the most common location of infantile haemangioma.
Pathogenesis of vascular malformations
Hyperplastic endothelial cells, characteristic in haemangioma, are absent in vascular malformations. Instead, these lesions are composed of dysfunctional architecture of vessels, which progressively enlarge (hypertrophy). The dysfunctional architecture includes abnormally developed vascular smooth muscle cells (glomous cells), irregularly enveloping the endothelium, leading to dilated, thin-walled dysfunctional vessels.
The formation of vascular malformations is associated with the vasculogenesis phase of early foetal development, at approximately weeks 6 to 10 of pregnancy. Vascular malformations are rare conditions, with the possible exception of sporadic (minor) venous malformations. Vascular malformations are sometimes associated with other congenital syndromes and then usually have a clear genetic fingerprint and inheritance track.
Still, the overwhelming majority of vascular malformations are sporadic, including lymphatic malformations, which have always been found to be sporadic. The pathogenesis of sporadic vascular malformations (irrespective of vessel type) remains a matter of speculation, but there is an increasing body of experimental evidence and data which eventually may develop into a well-formed idea about the pathogenesis of vascular malformations.
Of the currently patchy picture of information, some is experimental and some is anecdotal. For example, vascular malformations affect males and females equally and respond to hormonal changes (puberty and pregnancy) with sometimes aggressive growth; similar behaviour has been described after mechanical trauma. Such properties may hold clues about the underlying mechanisms of these sporadic lesions.
Biochemical research is pointing to faults in vasculogenesis as an important factor. A significant percentage of cases of all venous malformations show mutations in the TEK gene on chromosome 9p. This mutation is responsible for disabling a particular receptor, TIE2. The TIE2 receptor plays a crucial role in vasculogenesis. These observations also tentatively explain some promising initial results of treating lymphatic malformations with an mTOR inhibitor (sirolimus): the TIE2 receptor is closely linked with the mTOR signalling pathway in cellular metabolism. A number of further ‘hot spot’ mutations have been identified and will add to the understanding of the pathological developments.
Further vascular growth factors have been found to be upregulated, which has given rise to the hypothesis that some or all vascular malformations may also have some proliferation (hyperplasia) properties (in addition to the commonly accepted hypertrophy behaviour). This idea is further supported by recent observations of a particular enzyme (metalloproteinase-9) in some vascular malformations. Venous malformations display increased levels of neural components (whereas lymphatic malformations lack these completely) and show the presence of progesterone receptors. The latter finding may be related to the common enhanced growth rates of vascular malformations at times of hormonal changes.
For some of the rare hereditary vascular malformations, causal changes to chromosomes and genes have been identified. The sometimes well known effects of these mutations may in the future contribute to plausible explanations of the underlying causes and mechanisms of sporadic vascular malformations. For the time being, however, very little is known about the pathogenesis of these sporadic lesions.