Infection
Contents
This section discusses a number of themes closely related to / around the general topic of infection. This section keeps a focus on the host. We will address
- the role of commensal (friendly) microorganisms
- unwanted effects of antibiotic drugs
- sepsis (which may develop from infections or other conditions and is a life-threatening inflammatory condition).
Normal oral, nasal and skin microflora
The human body is colonised by commensal microorganisms, with numbers so great that they outnumber the cells of the body. These microorganisms, mostly bacteria, generally have no direct positive or negative effect on the host under normal circumstances. However, as part of the body’s normal microflora the commensal microorganisms benefit from nutrients and a hospitable environment provided by the host. In turn, they play an important role for the bodily functions of the host, for example vitally in digestion, or as part of the body’s defence mechanisms, the immune system.
Microflora
The commensal microflora are the microorganisms present on the linings of hollow structures and on the surface of the body. These inner and outer body surfaces are covered by epithelial cells and are unprotected from the external environment (see Figure 1).
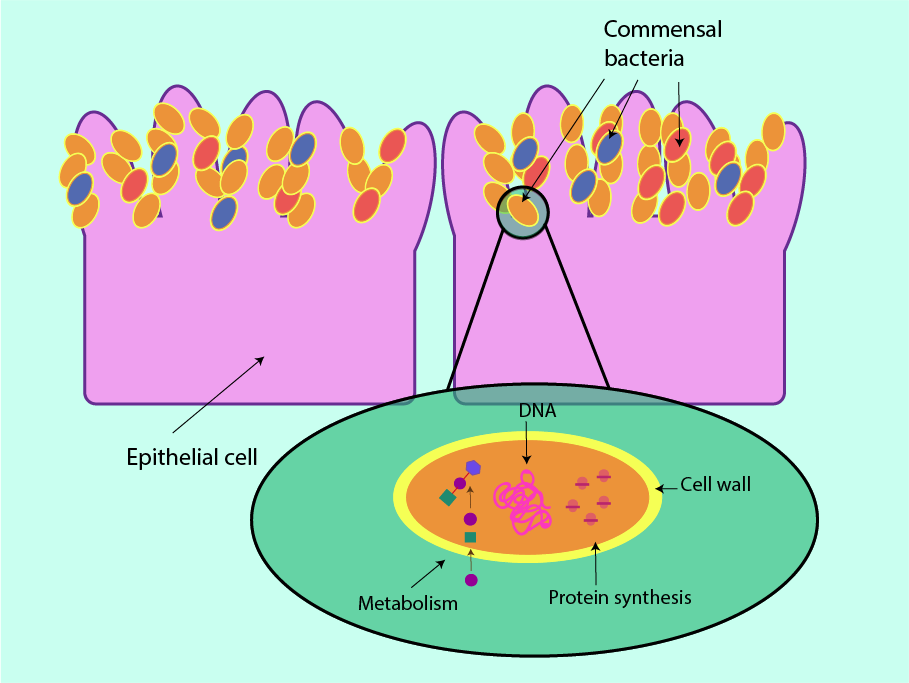
As the commensal bacteria evolved alongside their hosts, they do not usually pose a threat to the health of the host. Because of their co-evolution with the host, commensal bacteria are not normally recognised as pathogens by the host’s immune system. Commensal bacteria are in balance (homeostasis) with each other and with the host. Different body regions typically host different colonies of commensal microorganisms, as every strain is uniquely adapted to suit its preferred location (and there are individual differences between hosts). Typically anaerobic microorganisms of the small intestine, for example make use of the stable temperature and rich supply of nutrients there. Simultaneously the host benefits from their presence in a number of ways. In particular, commensal bacteria in the small intestine aid with digestion of plant matter which we would otherwise be unable to consume.
The maxillofacial region typically is host to a number of aerobic and anaerobic bacteria, Gram-positive as well Gram-negative species. For example, staphylococcus is uniquely adapted to live on the skin, it is able to cope with the high salt content of sweat and even uses sweat as a nutrient source.
Skin flora colonies typically include
- staphylococci
- streptococci of the viridans group
- Gram-positive and Gram-negative diphtheroids
- candida albicans
- malassezia furfur (common fungus species, can cause a range of skin infections and inflammation, including seborrhoeic dermatitis).
Oral flora colonies typically include
- alpha-haemolytic streptococci
- non-haemolytic streptococci
- Gram-negative cocci
- actinomyces species
- actinobacillus actinomyetemcomitans (particularly relevant in periodontal disease)
- Gram-negative anaerobic rods
- Spirochaetes (group of bacteria causing, for example, Lyme disease)
- candida species.
Nasal cavity and paranasal sinuses are typically colonised by
- Gram-positive rods
- Gram-positive cocci
- Gram-negative cocci, for example neisseria (large group of bacteria, including species that can cause meningitis).
Specific and non-specific host defence mechanisms exist to prevent microbial infection. These defence mechanisms are well developed in the head and neck region and include: an intact epithelial surface; nasal mucus, saliva and tears, all of which have antimicrobial properties. The commensal microflora also acts as an important non-specific defence mechanism (see Figure 2).
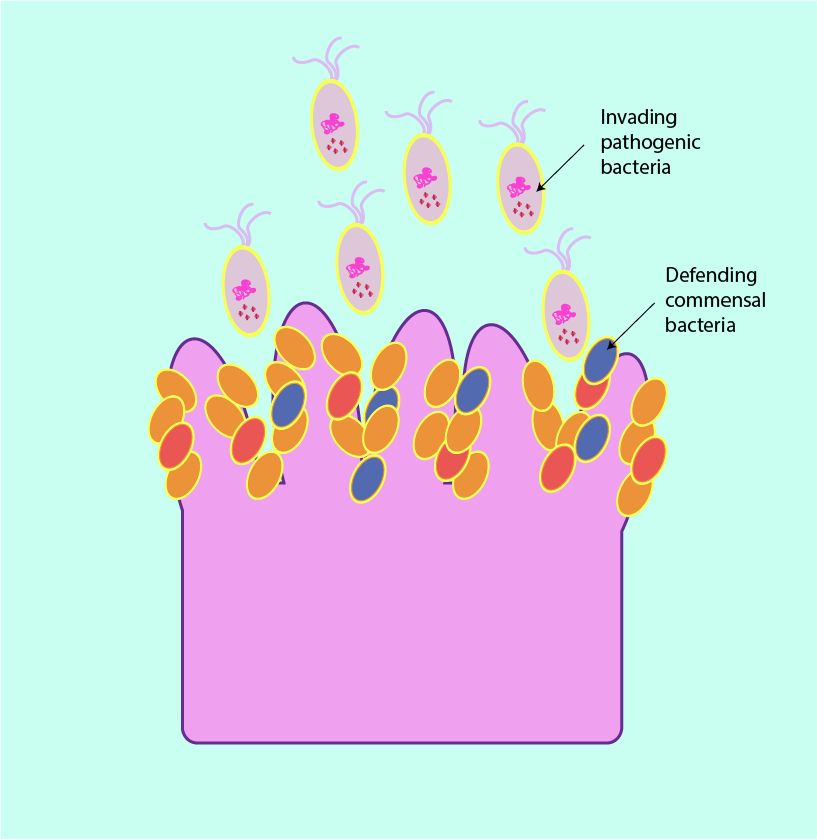
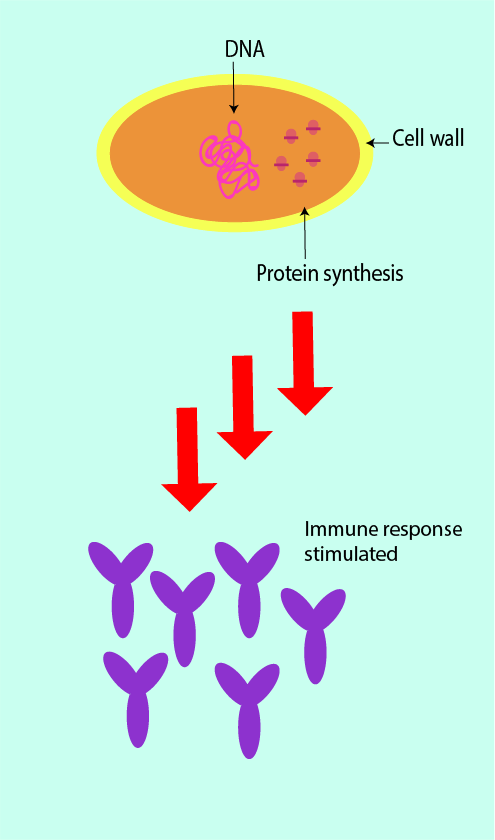
In addition, the commensal microflora also have a part to play in the immune response. They have generally immune-activating properties which stimulate the innate (non-specific) and adaptive (specific) immune defence mechanisms pathways when confronted with invading pathogens (this is symbolised in Figure 3).
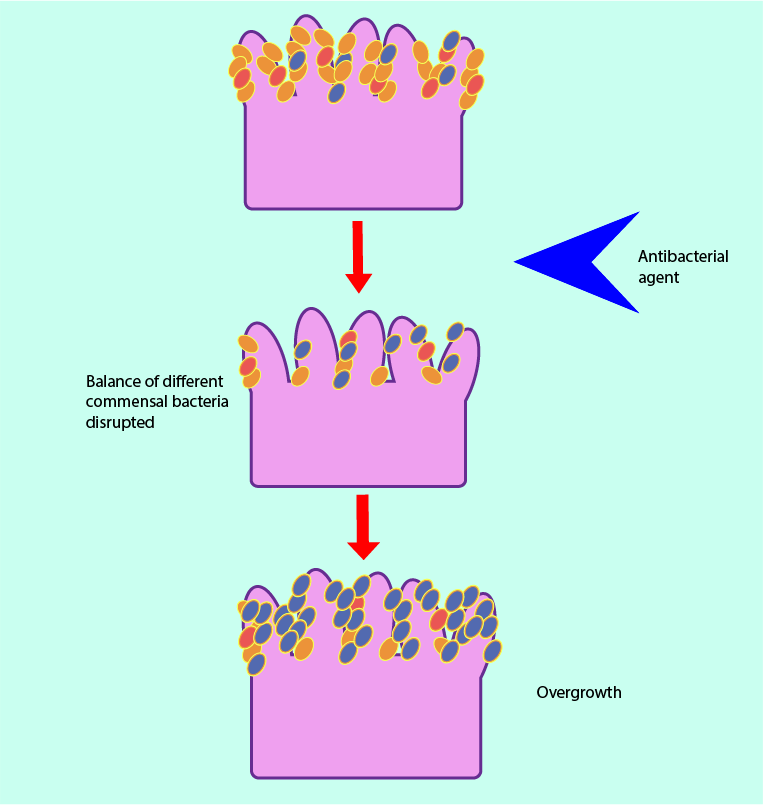
If the commensal microflora is altered, overgrowth of certain organisms can occur, leading to symptomatic infection (opportunistic infection). This is exemplified by the frequency with which candidal infection occurs following broad-spectrum antibacterial therapy. Broad-spectrum antibacterial agents not only damage their targets, they also harm commensal bacteria, leading to an imbalanced microflora (dysbiosis). This creates opportunities for overgrowth by other pathogens (this mechanism is illustrated in Figure 4).
Commensal bacteria can interfere with the immune system of the host in further ways. Slight misalignments and misregulation of the delicate balance of the immune system can trigger an exaggerated immune response of the host to commensal bacteria, for example giving rise to an overexpressed inflammatory response. On the other hand, the immune system’s mechanisms allowing for the co-existence of host and commensal bacteria may lead to pathological infections if commensal bacteria enter body regions where they are usually not found (for example, by trauma).
These aspects are relevant in not only the treatment of traumatic facial wounds but also generally in oral surgery as the oral cavity cannot be sterilised. In consequence, oral surgery will always lead to bacteraemia (presence of bacteria in the blood). This may be unproblematically resolved by an intact immune system in otherwise healthy people. However, people subjected to major and lengthy maxillofacial surgical procedures, people with a compromised immune system, or with a foreign body implanted (for example, a prosthetic joint or heart valve) are at risk of developing potentially serious infections following surgical interventions and will require risk assessment and possibly prophylaxis before (as well as during and after) surgery.
Unwanted effects of antibiotic agents
Side effects of antibacterial agents
Antibacterial agents often are the first-line, sometimes the only crucial treatment for bacterial infections, sometimes in combination with surgical interventions. The working principles of antibacterial agents aim to take advantage of the specific properties of bacterial cells and their metabolism where these differ from the host’s cell properties and metabolism. Still, like any form of systemic medication, antibacterial agents will have unwanted side effects on the host. Such unwanted effects can occur during treatment but may also extend beyond the treatment period.
The wanted effects of antibacterial agents in harming harmful bacteria can be roughly subdivided into four groups of mechanisms, affecting the bacterial cell wall, metabolism, protein synthesis or DNA.
Unwanted effects of antibacterial agents can be roughly categorised according to the same four classes of working mechanisms. Below is a non-exhaustive list of some of the general side effects from systemic treatment with antibacterial drugs within these categories.
Generally speaking, all antibacterial drugs have a negative impact on the entire gastrointestinal (digestive) tract, especially when taken orally. This is mainly because of their disruptive effect on the helpful and necessary bacterial microflora of the intestines. As a result, vomiting, abdominal pain, bloating and indigestion as well as diarrhoea and loss of appetite are common side effects of antibacterial drugs. It is also common for many antibacterial drugs to trigger a range of different allergic reactions of variable severity.
Side effects of antibacterial agents that affect the bacterial cell wall (for example penicillin and its many derivatives) include
- anaphylaxis (a fast and serious, potentially life-threatening allergic reaction)
- Stevens-Johnson syndrome (a protracted allergic reaction with flu-like symptoms and progression to a rash and blisters)
- urticaria (an allergic skin reaction, also known as hives)
- acute exanthemic pustulosis (a rare, severe allergic reaction that affects the skin and other organs).
Side effects of antibacterial agents that affect the bacterial metabolism (for example sulphonamides) include
- generally high allergy rates (‘Sulfa allergy’; most susceptible are people with slow metabolism and with long-term viral infections)
- drug hypersensitivity syndrome (severe, delayed allergic reaction that affects skin and other organs)
- hypoglycaemia (low blood sugar levels caused by stimulated insulin release)
- blood dyscrasias (a range of haematological conditions with deviations from the normal composition of blood).
Side effects of antibacterial agents that affect the bacterial protein synthesis (a wide range of different drugs, including macrolides (erythromycin) and clindamycin) include
- potentially severe disturbances of the gastrointestinal system (including ulcerative colitis)
- pro-arrhythmic action (increasing or decreasing heart rate, or causing irregular heart beat)
- hepatotoxicity (toxic effects on the liver as this class of drugs is metabolised in the liver)
- effects on respiratory system (changing quantity and quality of mucus in the respiratory system)
- allergic skin reactions
- rarely hearing loss.
Side effects of antibacterial agents that affect the bacterial DNA (for example metronidazole) include
- interactions with alcohol and other drugs
- disturbances of the gastrointestinal system
- otherwise only rarely associated with severe unwanted effects.
Side effects of antifungal agents
Largely similar to the unwanted effects of antibacterial agents, antifungal agents are usually likely to cause some stomach complaints, nausea, flatulence and diarrhoea. Allergic reactions are also common and may result in swelling of the face and airways (in severe cases) and/or skin rashes.
Side effects of antiviral agents
With the working principles of antiviral drugs being distinctly different from those of antibacterial and antifungal drugs, it is not surprising that also the unwanted effects of antiviral drugs are different and mainly develop over extended treatment periods. Side effects commonly consist of flu-like symptoms and some psychogenic effects ranging from milder forms (irritability) to more severe forms (including depression). There may also be long-term impacts on the haematological system, such as haemolytic anaemia.
Sepsis
Some general facts
Sepsis (formerly also called, and confused with, septicaemia or ‘blood poisoning’) is a serious, life-threatening inflammatory condition. It isn’t a very helpful term as it immediately conjures up the thought of overwhelming infection – which may or may not be a cause.
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Trauma, haemorrhage or burns may also be underlying causes for sepsis. Pathogens causing infections that lead to sepsis can be bacteria, viruses, fungi or other parasites; bacterial infections are the most common. The very young (new born babies in particular), the elderly, and people in generally poor health as well as those with a compromised immune system are most vulnerable to sepsis.
Not every aspect of the causes and development of sepsis is completely understood. It is difficult to understand how and why at some stage the immune system switches from fighting infectious pathogens to instead attacking and damaging the body’s tissues and organs. It has long been known that the immune response is a ‘two-edged sword’ that can harm both host and invader but why it should have such an extreme effect is puzzling. It is likely that the beginning of sepsis is triggered by an immune system response to combined host and pathogen factors. There is also some heterogeneity with different levels of over- or underactivity of the immune system that may occur at different stages of the disease (and with different people).
Mortality related to sepsis remains stubbornly high, with nearly half of patients with sepsis or septic shock (a useful definition of the word ‘shock’ used in this context is a failure of oxygen delivery at a cellular level) still dying in 2016 and sepsis being the primary cause of death from infection. The morbidity of survivors is also considerable, with, for example, about a third of adult sepsis survivors over the age of 60 not having returned to independent living after 6 months, and many suffering a variety of long-term problems.
It is of key importance that sepsis is not only promptly recognised and distinguished from less severe infections but that investigations, treatment and monitoring are started immediately. With this in mind, in 2016 the 3rd International Consensus about definitions for sepsis and septic shock has been agreed. The aim is to improve clinical management and outcomes for sepsis.
Signs and symptoms of sepsis
Infection is associated with a localised inflammatory response. There may, however, be some viable pathogens (bacteria, fungi, viruses, other parasites) present in the blood stream, most likely bacteria.
A combination of some or all of the following symptoms is called systemic inflammatory response syndrome (SIRS; with or without identifiable or identified source of infection):
- body temperature > 38 °C or < 36 °C
- accelerated heart rate (tachycardia) > 90 beats / min
- increased respiratory rate (tachypnoea) > 20 breaths / min
- abnormally high or abnormally low white blood cell count.
SIRS in conjunction with presumed or proven infection used to be called sepsis; sepsis in conjunction with signs of acute organ dysfunction (such as hypotension (reduced blood pressure), reduced urine output, reduced oxygen saturation, and a range of biochemical markers) used to be called severe sepsis. Severe sepsis in conjunction with persistent hypotension, even after adequate fluid replacement, used to be called septic shock.
The definitions as revised in 2016 do not distinguish sepsis and severe sepsis anymore, septic shock is defined as a sub-set of sepsis with need for vasopressor treatment in addition to adequate fluid replacement in order to increase the mean arterial blood pressure to > 65 mmHg and maintain lactate levels. This, of course, uses treatment modalities to define a pathophysiological condition which seems a little backward. The 2016 definitions also made recommendations to replace the SIRS criteria (as they have some shortcomings) by a slightly different system – but the newly proposed scheme has already been criticised and is likely to be changed again. The SIRS system is clinically well established and its use is continued.
Underlying pathophysiology of sepsis
The immune system is the body’s general defence mechanism. An appropriate response of the immune system to infection will eliminate the invading pathogens without harming the body tissues and organs. The immediate response to infection comes from the first layer of defence, the so called innate immune system (all living creatures have such a defence system). It provides an immediate, non-specific response and triggers (local) inflammation by the release of pro-inflammatory compounds and activates cellular responses. The second layer of defence is called the adaptive immune system which provides a delayed but more specific reaction to pathogens (only vertebrates have such a second layer of defence) and also acts as a long-lasting memory of infection in the body’s immune system.
There are two main pathological strands underlying the development of sepsis: overproduction of pro-inflammatory mediator molecules (cytokines) leading to organ-damaging responses and loss of regulation of the normal blood clotting balance, alongside further cellular dysfunction resulting from these two pathological mechanisms combined. The inflammatory and coagulation systems are generally closely related in regulating and maintaining body functions. It is also fair to state that the systemic inflammatory response in sepsis is not easily classifiable as a suppressed or overactive immune response – it can be argued that an initially underactive response from the immune system to infection, failing to eliminate the pathogens swiftly, logically mandates a much ramped-up response in the attempt to eliminate the infectious pathogens. It is even controversial if excessive inflammatory response is the main contributor to high mortality from sepsis and clinical trials using aggressive administration of anti-inflammatory agents did not lead to improved survival. Clearly, sepsis is a multi-factorial condition.
The combined effects of dysregulation of inflammatory response and coagulation cause multiple organ and tissue damage. Overstimulation of the production of pro-inflammatory compounds (cytokines, tumour necrosis factor (TNF) and a number of interleukins) all contribute to severely disturbed haemostasis. Normally, there is a very fine balance maintained between the flow properties of blood and coagulation. An initial response to overstimulation of pro-inflammatories is increased coagulation, leading to blood clots in the ‘wrong’ locations in the vascular system and thus causing tissue necrosis from lack of blood supply in the affected areas. On the other hand, damage to the vascular system from high levels of pro-inflammatory compounds in the system also leads to bleeding at specific sites because not enough coagulation-regulating factors, white blood cells and platelets, are in circulation. This seeming contradiction of simultaneously too high and too low levels and rates of coagulation (Disseminated Intravascular Coagulation, DIC) simply results from different local ‘interpretations’ of the unbalanced state of the overall coagulation system. Much experience of the vascular management is gained from obstetric bleeding. It is incredibly difficult.
The entire circulatory system is affected and resulting changes to the endothelium (especially the lining of the blood and lymphatic vessels) from the inflammatory response are related to organ damage, including widespread tissue oedema. Often lethal organ damage to, and failure of, the lungs, kidneys, nervous system and liver occur and may develop to so called multi-organ failure, often worsened by additional infection(s).