Ketamine
Ketamine was developed as an anaesthetic agent in the 1960s (Figure 1). Since then it has been used widely.
Ketamine is used as a recreational drug for its hallucinogenic effects, although depending on dosage, it has been reported to have both stimulatory and sedating effects. It has considerable addictive potential and a number of adverse effects arising from short- and/or long-term use. Ketamine is used in general anaesthesia for a number of applications, in particular in trauma surgery. Other, more recent applications include the management of severe depression, chronic and neuropathic pain, and as an adjuvant analgesic, for example in palliative care, in order to reduce opioid dosage, or perioperatively in orthopaedic surgery to prevent or reduce postoperative pain.
Most anaesthetic agents, such as propofol and barbiturates, work by activating GABAA receptors. The complicated physiological effects of ketamine are different, and are strongly dose-dependent. Ketamine belongs to a class of anaesthetic agents called NMDA (N-methyl-D-aspartate) receptor antagonists (Figure 2). These agents inhibit the function of the NMDA receptor and induce a state termed dissociative anaesthesia, referring to the hallucinogenic effects of these agents.
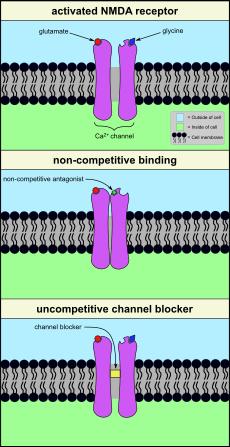
NMDA receptors regulate the function of Ca2+ ion channels, required for neural signal transmission in the central nervous system (brain and spinal cord). For signal transmission, the ion channel needs to be open. For this activated state, it is necessary that glutamate and glycine are bound to their respective receptor sites. There are several different ways in which the function of this ion channel can be hindered, by inhibiting the function of the NMDA receptor. One possibility is to competitively block the binding sites of glutamate and/or glycine by some other agent. A NMDA antagonist can achieve inhibition of the receptor by non-competitive binding to some other site of the receptor (Figure 2, middle). This is the working principle of a number of anticonvulsive agents such as gabapentin which also have a neuropathic analgesic effect. Other agents may simply block the calcium ion channel (Figure 2, bottom). Ketamine belongs to this category of uncompetitive channel blockers, as do alcohol and nitrous oxide (‘laughing gas’).
Ketamine mainly interacts with the NMDA receptor channel, but in higher dosage it interacts with many other structures (including other ion (Na+) channels, opioid and GABAA receptors). This explains some of the adverse effects of ketamine, as well as some of its beneficial effects. Despite the long history of use of ketamine, its complicated physiological effects are only partially known. A more recent avenue is the use of S(+)-ketamine in emergency surgery and intensive care: like many other organic molecules, ketamine (Figure 1) exists in two forms (isomers) that are mirror images of each other but are otherwise chemically identical. Receptors and their binding sites often respond slightly differently to the geometrically subtly different isomers of agent molecules. Accordingly, preparations of agents in a ‘pure’ form with only one of the two isomers may have advantages, such as better efficiency (tramadol is an example of an analgesic agent that interacts with opioid receptors and is used as a mixture of its geometric isomers).
Dosage-dependent effects are common for agents that mainly interact with NMDA receptors. Think of the difference of consuming a small amount of alcohol and the effects of binge drinking. The anticonvulsant agent gabapentin is a powerful medication for treatment for epilepsy, whereas gabapentin in very low doses can be helpful to treat neuropathic or chronic pain. For ketamine, relatively higher doses are the regimen of anaesthesia, whereas low doses are used in the treatment of chronic pain and severe depression, and some low-dosage schemes are being explored in the context of schizophrenia management.