Anaesthesia
Contents
We briefly discuss some aspects of general anaesthesia in more detail than our previous pages. Part of our discussion deals with surgery under general anaesthesia in general, part of our discussion is more specific for oral & maxillofacial surgery. Our topics include
- some thoughts about the weird state of general anaesthesia;
- the stages of general anaesthesia;
- monitoring and maintenance during general anaesthesia;
- protection and maintenance of the airway.
Some thoughts about the weird state of general anaesthesia
General anaesthesia is undoubtedly the enabling technique for major surgery. The term anaesthesia literally means ‘without feeling’, but provides little insight into the state of general anaesthesia. Is it a state of unconsciousness, or a state of unresponsiveness, possibly combined with a degree of amnesia? Given that it remains unknown how exactly consciousness is established in the brain, and there is a lack of methods to assess precisely levels of consciousness, it is even more remarkable how safe, effective and successful general anaesthesia actually is. As early as 1950 general anaesthesia was described as a triad of three key concepts:
- relaxation – reduced movement of muscles;
- analgesia – lack of sensation of pain;
- narcosis – state of unconsciousness.
There is a considerable body of investigations about various different aspects of the mechanisms involved in general anaesthesia, accompanied by a large number of hypotheses and ideas about the relationship between consciousness and general anaesthesia, yet the overall picture remains a little patchy.
The biochemical and physiological properties of most drugs used in anaesthesia are reasonably well understood. Anaesthetic agents alter neuronal activity by hyperpolarising neurons and in this way decrease excitation patterns similar to those observed in non-rapid-eye-movement sleep phases (as observed by EEG (electroencephalogram) measurements. Low doses of sedatives lead to drowsiness and disorientation, similar to drunkenness. At higher doses, a person becomes unresponsive to command. At this point, somebody is considered unconscious. This old-fashioned behavioural definition of unconsciousness is convenient but unsatisfactory. Unresponsiveness can occur without unconsciousness (for example, when one is dreaming). Paralysing agents used during general anaesthesia do not suppress consciousness. Some agents (such as ketamine; see below) simply impair somebody’s willingness and/or ability to respond to a command/question. Depending on dosage, the inability to respond may also occur as a consequence of impairment of working memory, with immediate forgetfulness. Retrospective oblivion thus is no proof of unconsciousness during general anaesthesia. Similarities with some frontal brain lesions that impair movement have been pointed out, where again unresponsiveness does not equate as unconsciousness.
However, somewhere on the spectrum from behavioural unresponsiveness to brain death, consciousness must vanish. EEG seems either not suitable as diagnostic measurement to identify this transition, or it is still insufficiently understood what to look for in an EEG to find that spot.
Reduced metabolism and blood flow activity of the thalamus region in the brain has been suggested as a switch to unconsciousness. In humans, lesions of the thalamus are associated with a vegetative state. Recovery from that state has been associated with restoration of the thalamus – cingulate cortex communication / activity (the cortex is a large brain region associated with multiple functions). Also, electrical stimulation of the thalamus improves behavioural responsiveness in minimally conscious people. However, thalamus activity does not decrease with all anaesthetics. For example, ketamine (see below) increases thalamus metabolism. So, thalamic activity alone does not explain consciousness. It is far more likely that thalamus communication with several cortical areas is the required feature to produce consciousness (functional disconnection of the thalamus during general anaesthesia has been observed by neuroimaging).
But which cortical areas are important in this communication network (each cortical sub-region is highly specialised)? There are several candidates, but no clear individual roles. Perhaps unconsciousness simply may be brought about by subtle changes of the dynamics of neural activity, enough to impair the brain’s ability to integrate information. Perhaps during deep general anaesthesia / unconsciousness, the cortico-thalamic system can still be active, even hyperexcitable, but may have lost its ability to produce its normal repertoire of responses. This would be a situation similar to convulsive seizures where consciousness can be lost despite high neural activity.
It is tempting to compare general anaesthesia with sleep, the only condition when healthy humans lose consciousness. Clearly, general anaesthesia is not the same as natural sleep, but similar patterns of deactivation of the brain arousal system have been observed. Evidence from such comparisons of general anaesthesia and sleep states suggests that the breakdown of connectivity between brain regions, basically a loss of information and/or its integration is responsible for unconsciousness. This idea recognises the fact that the brain is a highly complex neural network, the function of which is defined by its multiple connectivities, rather than by isolated functions in / of isolated regions. The degree of integration of the system is responsible for the richness of its responses and the information generated. All these interconnected agents together act as a single entity, or as one could say: the whole is more than the sum of its parts. It is feasible to think about ways to use EEG measurements to evaluate degrees of consciousness based on this connectivity model. The model also suggests that one should be very careful in interpreting a lack of responsiveness, and perhaps more importantly it implies that consciousness is not a two-state on/off condition, but falls on a spectrum (consistent with observations of degrees of sedation). Abrupt loss of consciousness at threshold levels of anaesthetic agents suggests that the repertoire of neural states that constitutes consciousness, collapses in a non-linear manner. Also, the effects of the ascending reticular activating system (the ‘vigilance system’, a network of neurons in the brain stem) are likely to be a relevant mechanism. It is thought to be the mechanism in rendering someone unconscious traumatically, which is clearly related as it is an unconscious state which may or may not be associated with identifiable brain damage.
The current state of understanding about general anaesthesia implies that consciousness requires a large repertoire of discriminable states (several such models of complexity have been developed). Anaesthetics could then produce unconsciousness either by preventing integration (blocking interactions between regions) or by reducing information (shrinking the number of activity patterns), or both. Complex-network brain models are also in line with clinical observations of slower recovery from general anaesthesia (or even temporary delirium) for older people. Much more research about the mechanisms of general anaesthesia is needed, but a powerful model / hypothesis is certainly a good start toward that goal.
The stages of general anaesthesia
There are usually three types of agents used for general anaesthesia (the triad of anaesthesia): a hypnotic agent, an analgesic, a muscle relaxant. Choice of components as well as their relative and absolute amounts depend on patient factors and on the planned surgery, with an individually optimised ‘cocktail’. This optimisation needs to consider the balance between general anaesthesia as a depressant and surgical trauma as a stimulant (although this can and often is reduced by concurrent use of local anaesthesia). General anaesthesia follows three stages
- induction (transition from awake to anaesthetised);
- maintenance;
- emergence.
The different stages of general anaesthesia require different composition and dosage of anaesthetic agents.
Induction of general anaesthesia can be achieved by intravenous injection or by inhalation of a volatile.
For intravenous induction, the most commonly used agent is propofol, often in combination with an opioid such as fentanyl for a smoother transition to unconsciousness. If intubation is required (in the UK for oral and maxillofacial surgery almost always), a muscle relaxant will also be included in the induction cocktail of drugs. Propofol is a fast- and short-acting hypnotic, its action is established typically within a minute and lasts for a few minutes. Physiologically, propofol interacts with GABA receptors and is a vasodilator, it is metabolised by the liver (with potentially more severe adverse effects in elderly and seriously ill people). Generally, the induction phase of general anaesthesia is a disruptive condition with multiple effects on body functions and organs, including hypotension, reduced airway reflexes, respiratory depression, unconsciousness, reduced tone of the oesophageal sphincter (risk of aspiration). These systemic effects lay out the task for the anaesthetist for intubation: the motto here is swift and gentle action (a rapid induction protocol is followed if regurgitation and aspiration are a concern). Ketamine is another, less commonly used intravenous anaesthetic that can be used for induction. Ketamine causes sedation, analgesia and bronchodilation along with other systemic effects. Its pharmacological mechanism of action is different from that of propofol in that ketamine acts as a stimulant on the sympathetic nervous system. Its main role as an induction anaesthetic is in trauma surgery (particularly on-site surgery due to its airway maintenance benefits), generally for people with hypotension, for those with severe asthma, and for children (who appear less likely to adversely react to the common ‘emergence phenomena’ – basically unpleasant (to adults) hallucinogenic effects) with congenital heart disease. Ketamine is more popular in US practice where oral and maxillofacial surgeons are trained in, and often provide their own office-based forms of general anaesthesia and deep sedation.
Induction by inhalation via a mask of a volatile agent most commonly uses sevoflurane, with increasing dosage of the agent (from 1 to 10 %) in a gaseous mixture of O2 (ca. 30 %) and air, or nitrous oxide. Volatile organofluoro-compounds such as sevoflurane (Figure 1) are exhaled largely unmetabolised.
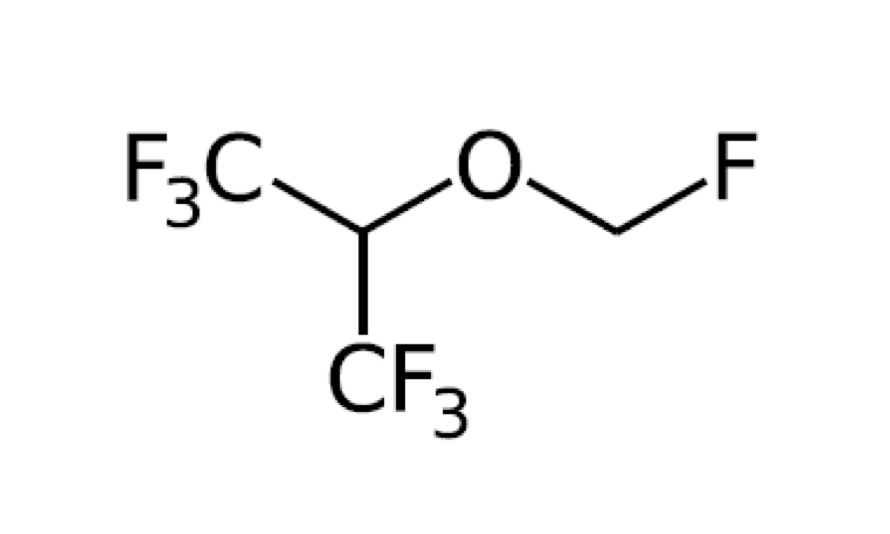
The mechanism(s) by which organofluoro-compounds act as hypnotics are only poorly understood. Similar to intravenous agents such as propofol, organofluoro-compounds have systemic effects on most organs. The effects include hypotension, dysrhythmia, reduced airway reflexes and respiratory depression, and – of course – unconsciousness. Induction by inhalation is commonly used for children in oral and maxillofacial surgery.
When muscle relaxants are required, there are two types of such agents in use. They belong to so called depolarising (suxamethonium chloride, which historically was the preferred choice for facilitating intubation due to its rapid onset and short-lasting effects, this has reduced because of its well-known adverse effects of post anaesthetic muscle pain; short acting agents such a mivacurium were introduced as an alternative to suxamethonium) or non-depolarising agents (atracurium besylate, or the aminosteroid rocuronium bromide). Both types of agents lead to paralysis by disabling the transmission of nerve signals across the neuromuscular synapses, by different mechanisms.
Common systemic analgesics used for induction of general anaesthesia are opioids such as ultra-short acting synthetic opioids remifentanil and alfentanil. In order to reduce the amount of systemic analgesia, it is common to use local injections of analgesics at the surgical site, commonly used agents are lidocaine with adrenaline (for haemostasis in oral and maxillofacial surgery) and bupivacaine or levobupivicaine.
Maintenance of general anaesthesia covers the period from the start of a surgical intervention to its completion. Maintenance is most commonly achieved by application of inhaled volatile anaesthetic agents, such as sevoflurane (Figure 1). Their equilibrium concentration at the site of action, the brain, has to be monitored continuously in order to have an idea about the depth of anaesthesia. Some people (smokers and regular consumers of alcohol) may require higher doses of anaesthetics. When use of muscle relaxants is continued, neuromuscular function needs to be monitored with a peripheral nerve stimulator (for example, checking the ulnar nerve (arm)). General anaesthesia can also be maintained intravenously, by continuous delivery of propofol with or without an opioid added. This form of maintenance minimises any hangover effects and nausea (adverse effect of organofluoro volatiles), so has a role particularly in day surgery, neurosurgery and for people who are known to be affected by severe postoperative nausea.
Emergence from general anaesthesia, restoration of consciousness, is essentially the reversal of induction. Maintenance agents are reduced or stopped. The recovery of neuromuscular function needs to be established; the reversal of the effects of non-depolarising muscle relaxants can be accelerated by reversal agents (acetylcholinesterase inhibitors). Adequate analgesia and prevention of nausea / vomiting postoperatively need to be ensured. In most cases, extubation is carried out when airway protection without intubation (or tracheostomy; see below) is not an issue. Some form of sedation has to be maintained temporarily when postoperative intensive care is required.
Monitoring and maintenance during general anaesthesia
Perioperative care has many different aspects, including
- positioning and repositioning - to avoid nerve injuries and other pressure damage under general anaesthesia; appropriate padding as required; repositioning is relevant for a number of maxillofacial reconstructive surgeries;
- maintaining body temperature - to avoid hypothermia under general anaesthesia; monitoring body temperature, use of warm air devices and blankets; calf intermittent compression to prevent coagulopathy that can be caused by hypothermia and/or static positioning;
- fluid balance - may include blood products as well as intravenous hydration; for longer interventions a bladder catheter will be placed;
- airway scrutiny - because of a shared work space between surgeon and anaesthetist in maxillofacial surgery, the anaesthetist needs to continuously monitor airway safety as tracheal tubes can easily become dislodged in these circumstances, endotracheal cuff pressure (whether tube or tracheostomy) is monitored to prevent tracheal mucosal necrosis;
- monitoring of vital parameters - such as blood pressure, body temperature, oxygen saturation, heart rate, respiration rate (this is usually controlled ventilation in longer procedures); application of medication(s) to control functions as and when necessary;
- monitoring depth of anaesthesia – to avoid awareness during anaesthesia; indirect assessment through observation of papillary dilation, sweating, lacrimation, or by monitoring of blood pressure, heart rate, concentrations of volatile anaesthetic agents;
- monitoring and adjusting analgesia – observation of sympathetic response, despite unconsciousness, and titration with analgesics accordingly.
Preoperative assessment plays an important role in preparing for optimal perioperative care. This is particularly relevant for maxillofacial patients with comorbidities and/or difficult airway situations.
Protection and maintenance of the airway
Given the need to share the workspace between anaesthetist and surgeon in oral & maxillofacial surgery, the most common approaches to protection and maintenance of the airway differ from those most commonly used in other areas of surgery.
Nasotracheal intubation
Nasotracheal intubation is the most common method for many oral and maxillofacial surgical procedures (intraoral and oropharyngeal interventions, including mandibular reconstructive surgery and other major maxillofacial surgery, dental surgery, orthognathic procedures). This method has the advantage of little interference with the oral anatomical structures and often is the preferred method when having to deal with a difficult airway, for example in the presence of trismus, or in children with craniofacial syndromes.
Swift (in order to avoid complications from aspiration of gastric acid) and gentle (in order to minimise epistaxis (nose bleeds) and sore throat) intubation, with an appropriately sized and lubricated endotracheal tube is the way for the anaesthetist to go about this method.
Contraindications for nasotracheal intubation include any blockages of the nasal airway, previous skull base fractures or suspected current skull base fracture, severe facial trauma (especially to the midface), a history of repeated severe epistaxis episodes, suspected epiglottitis.
Orotracheal intubation is the alternative to nasotracheal intubation. It is easier to perform and alleged to be less traumatic by anaesthetists less skilled in nasotracheal intubation (this can, of course, be true but is dependent on the skill of the anaesthetist and the nasopharyngeal anatomy of the person being intubated).
Submental intubation is basically oral intubation but the tube is brought out of the patient via a small incision in the floor of the mouth and a skin crease incision just under the jaw line in the submental triangle of the upper neck (see below).
Tracheostomy
Openings into the airway are termed tracheostomy (permanent or temporary) and cricothyroidotomy (only ever temporary). It bypasses the upper airway passages (nasopharynx and oropharynx) if they are obstructed or being operated on in such a way that a naso- or orotracheal endotracheal tube cannot be used. It has the advantage of reducing the ‘dead space’ (an area where O2/CO2 to haemoglobin exchange cannot take place) and can protect the lungs from substances that are noxious.
It may be used in a variety of conditions but in relation to maxillofacial surgery, this is usually head and neck cancer or major trauma. In these circumstances, there are mainly two reasons to place a, usually temporary, tracheostomy:
- when endotracheal intubation throughout major surgery is not an option (considering the shared space between anaesthetist and surgeon);
- to be able to deal safely with major post-operative swelling.
While securing an airway is important in advanced cervicofacial infection, surgical airways should be avoided when possible as they carry a higher risk of infected major pulmonary and mediastinal complications. A permanent tracheostomy as part of a total laryngectomy is not covered here. However, in a maxillofacial surgery context, a permanent tracheostomy may be the ultimate method to prevent aspiration in cases of severe dysphagia.
A tracheostomy is a surgical opening into the trachea to secure a patient’s airway. It allows the passage of a cuffed tube into the airway which ensures that nothing such as vomit or blood can pass into the lungs, and only what is passed through the tube (oxygen or air) can pass into the lungs.
A tracheostomy is usually performed in theatre and involves the following steps:
- position patient: supine with neck hyperextension using a sand bag and head ring;
- clean area with appropriate antiseptic;
- drape leaving tunnel for access (in maxillofacial surgery, an endotracheal tube is often already in place, so leave space for the anaesthetist);
- infiltrate area with lidocaine 2 % (analgesic) and 1 : 80,000 diluted adrenaline (vasoconstrictor);
- incision through skin, usually horizontal for scar aesthetics (if temporary as is usually the case in maxillofacial surgery);
- split infrahyoid strap muscles in midline;
- expose, retract or divide thyroid isthmus (this is very operator dependant, there is no absolute right or wrong way to do it);
- expose and confirm identity of trachea, visually and digitally;
- choose site of tracheostomy, usually the second tracheal ring (avoid cricoid cartilage);
- alert anaesthetist;
- check tracheostomy tube and fittings (there is dispute about checking cuff inflation, but this would seem a sensible check before placing the tube);
- incise trachea; there are different schools of thought on whether this should be a simple vertical incision, a hole or a caudally based flap (’Bjork flap’);
- ask anaesthetist to withdraw endotracheal tube but leave visible;
- introduce tracheostomy tube, complete haemostasis;
- secure the tracheostomy tube – it is common to suture this to the surrounding skin especially during long operations;
- close subcutaneous tissues;
- close skin edges.
A cricothryroidotomy is a similar process where the cricothyroid membrane (above the first tracheal ring) is penetrated; usually as an emergency surgical airway although data exists to support its role in short term elective use for both airway maintenance and suction. The device is actually designed for lung toilet, not airway maintenance.
Percutaneous tracheostomy is a process by which a needle puncture is made via the skin into the trachea at the level of a conventional tracheostomy. The puncture is dilated by a series of instruments or a progressively thickened instrument (‘the rhino horn’). It is used most often in intensive care, although some surgeons have been converted to the technique (the author is not one of them).
Given that most tracheostomies in maxillofacial surgery are temporary, it may be helpful to be familiar with the concept of a tracheostomy (see above) and what is involved in the process to decannulate, clean or remove a tracheostomy on the ward. Decannulation is followed in a stepwise process:
- When the patient’s post-operative recovery has reached a stage whereby the upper airway problem has resolved, and the removal of the tracheostomy can be commenced.
- A fenestrated tube is used first to encourage the patient to use their upper airway again, ready for decannulation.
- This can be assessed by the patient’s tolerance of occluding the tube using a decannulation button.
- Generally, on the ward the patient is monitored for 24 to 48 hours with the button in place, observing the oxygen saturation levels of the patient to ensure they are well perfused and there are no breathing difficulties.
- Once the patient can maintain their own airway, remove the entire tube.
- The stoma site is then covered using an airtight dressing. The patient should be advised to press on the dressing when talking or coughing to prevent air being forced through the healing stoma.
- The stoma site will normally take approximately two weeks to close and heal up, occasionally some patients require stitches.
When a patient is ready to have their tracheostomy tube removed, or if the tube needs replacing, the following is a common step-by-step guide to removing and changing a tracheostomy tube.
Step 1: Check all equipment – including inflating cuffs and inserting & removing inner tubes and introducers.
Step 2: Sit the patient in an upright position, with the neck extended and suction the airway in preparation.
Step 3: Clean around the stoma with sterile saline to remove any encrustation.
Step 4: Deflate the cuff if present, and ask the patient to breathe in and then, as they breathe out, remove the tube.
Step 5: Insert the new tube following the passage with the introducer in place, asking the patient to take a deep breath in first, then breathe out and hold breath out.
Step 6: Remove the introducer.
Step 7: If using a tube with an inner tube, insert the inner tube and inflate the cuff if necessary.
Step 8: Feel air being moved in and out of the tube on the back of your hand and then secure the tube, usually with the ribbons accompanying the tube.
Submental intubation
If nasotracheal intubation is impossible and there is a low risk that prolonged ventilation or repeated surgical interventions will be required and there is no expectation of major post-operative swelling (which would necessitate tracheostomy), submental intubation can be an alternative method. It is less invasive than a tracheostomy and is compatible with surgical repair of midfacial fractures. Submental intubation involves an incision to create a pathway between the skin and the floor of the mouth through dissection through the mylohyoid (pair of muscles along the neck). As this method involves the oral cavity, it is not suitable for the surgical repair of comminuted mandibular fractions. Where submental intubation is an option, it compares favourably with tracheostomies regarding complications and morbidities, and smaller, less visible scars result from this method of intubation.