Other drugs
The biochemical and physiological effects of drugs are, obviously, independent of their legal status. For example, if a substance has potential to lead to addiction, it has these basic properties whether prescribed and used therapeutically or bought illegally and used recreationally. The three main categories of widely used drugs, other than nicotine and alcohol, that may be causing problems when faced with oral or maxillofacial conditions and/or surgery are i) opioids, ii) tranquilizers, and iii) stimulants.
Opioids
Opium extracted from poppy seeds has been used as a medicinal and a recreational drug for thousands of years in the Middle East, in ancient Egypt and Greece, in Europe. Poppies have long been known for their analgesic effects: the Latin name papaver somniferum means ‘sleep-bearing poppy’.
In the 20th century the active ingredients in poppy extracts (such as morphine) or cannabis (tetrahydrocannabinol, THC) have been chemically identified and fully characterised. Advances in chemical technology as well as in understanding the physiological properties of these compounds have made a host of similar synthetic compounds available.
Opioids all act upon the same group of receptors within the human central nervous system to produce their effect, the opioid receptors. These receptors already exist to accommodate naturally occurring compounds produced by our own bodies to relieve pain (endorphins and enkephalins). These compounds are the reason why athletes, such as marathon runners and rugby players, are unaware of pain until after their goals are achieved. Broken bones have been known to go unnoticed for a time thanks to the analgesic effects of these powerful endogenous compounds. With synthetic opioids interacting with the same receptors, it is unsurprising that their medicinal use is predominantly pain relief and anxiolysis/sedation. Where opioids are used as recreational drugs it is mostly for the ‘high’ (the anxiolytic and sedative effect) that usually accompanies (or even dominates) the pain relief effects.
Whether a drug is legal or illegal is of little importance for their biochemical functions, especially given that often legal and illegal opioid substances have very similar structures and, therefore, similar effects. Figure 1 shows just how chemically closely related some of these drugs are.
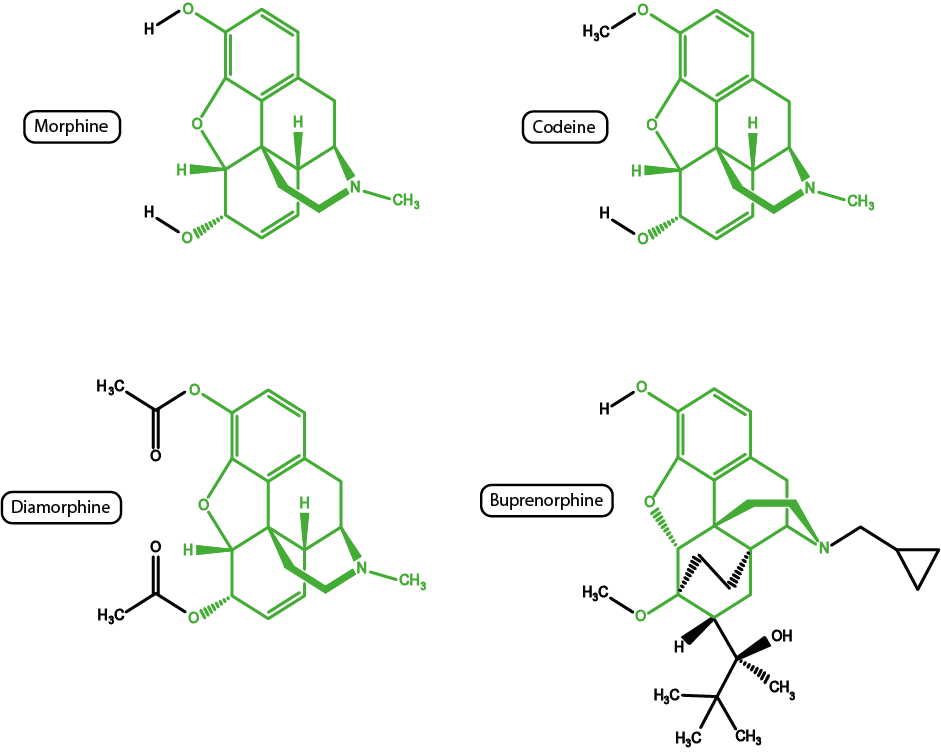
Codeine (see Figure 1) is a pro-drug that first needs to be converted to its active metabolite morphine by the body. Not all people can metabolise codeine (perhaps 80% of the population), hence in some cases codeine fails to have an analgesic effect. All of the opioid substances shown in Figure 1 (plus many more not shown in Figure 1) share many of the structural features of the morphine molecule. Given the similarities in structures, there are also similarities in properties. For example, diamorphine (probably better known as heroin) is a much more powerful analgesic (and is more soluble, allowing tiny quantities to be delivered) than morphine but, unfortunately, also has much enhanced addictive potential.
Buprenorphine is another powerful analgesic in clinical use; the substance has some practical advantages in that it can be prepared and administered in many different ways. Unfortunately as an agonist/antagonist it is only partially reversible by naloxone (a drug that is generally used to counteract the effects of opioids, especially for overdosing).
Another opioid very closely related to morphine is oxycodone. In the UK it is sometimes used as an alternative to morphine in palliative care: oxycodone has similar analgesic properties but is sometimes better tolerated with regard to poor kidney function and leads to less problems with drowsiness and hallucinations.
Figure 2 shows two of the (very many) components of cannabis, tetrahydrocannabinol, THC, (top) and cannabidiol, CBD, (bottom).
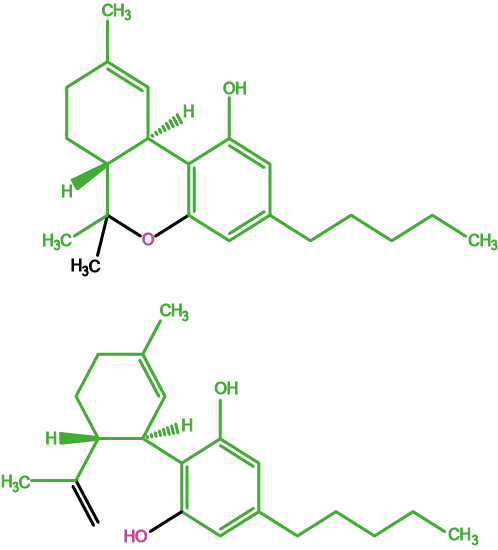
The two molecules have similar structures but only THC has pleasant (relaxing) psychoactive and/or analgesic effects. CBD is not psychoactive but is strongly suspected to hamper cognitive abilities. Natural cannabis products with enhanced THC concentrations (from many years of selective breeding of more potent cannabis plants) usually also have higher CBD concentrations. This may explain increasing numbers of adverse effects with the usage of cannabis as a recreational drug in the recent past. Medicinal cannabis preparations contain controlled concentrations of THC extracts from the plant material.
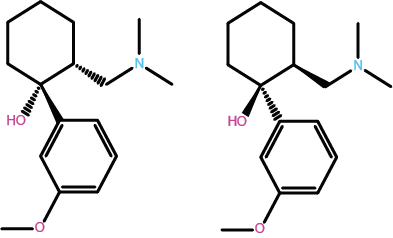
Figure 3 depicts the molecular structure of a very widely prescribed opioid, tramadol, used for the treatment of mild to medium pain levels. Tramadol is a less powerful analgesic than morphine but has similar addictive properties (partly explaining the recent black-market epidemic of tramadol, especially in the US). Tramadol is something of a peculiarity amongst medicinal drugs: Figure 3 depicts two geometric isomers (versions) of the molecule. Normally, only one particular form / isomer of a molecule is biochemically active. For tramadol, the two isomers enhance each other’s effect.
Fentanyl is commonly used to control chronic severe pain, often applied via skin patches, ensuring a steady and stable systemic concentration of the drug. The molecular structure of fentanyl is shown in Figure 4.

Fentanyl is a more powerful analgesic than morphine. When used ‘as intended’ its addictive potential is very rarely problematic as the pain-control effect is then by far the most noticeable effect. However, fentanyl used as a recreational drug is infamous for being involved in the untimely deaths of some celebrities.
Tolerance to analgesic and euphoric effects of nearly all opioids occurs relatively rapidly, whereas sedative effects on the respiratory centre occur more slowly. Therefore, when using opioids as recreational drugs, an increase in dose to reach the desired ‘high’ can potentially lead to fatal respiratory depression. Despite having repeatedly mentioned the addictive power of nearly all opioids, if used as analgesics under medical care conditions, the danger of developing a dependence is much less than is often feared.
Tranquilizers
Similar to opioids, tranquilizers also cause their effect through interactions with specific receptor sites in the brain. We discuss benzodiazepines as an example. They are a common class of tranquilizers. Benzodiazepines act upon the GABAA receptors in the brain. This is illustrated in Figure 5.
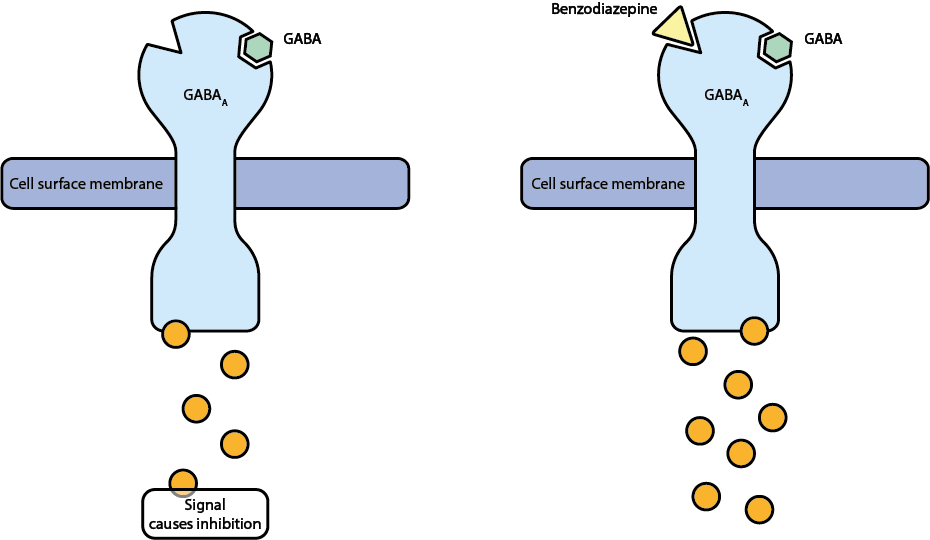
Normally the GABAA receptors interact with the neurotransmitter gamma-aminobutyric acid (GABA). GABA has an inhibitory effect, preventing nerve impulses. Benzodiazepines fit into a different site on this receptor, intensifying its inhibitory action. Changing the chemical structure of these molecules causes slightly different effects as the molecule will then interact slightly differently with the receptor. However, the core of the molecules (the pharmacophore) remains the same, giving similar overall effects (see Figure 6).
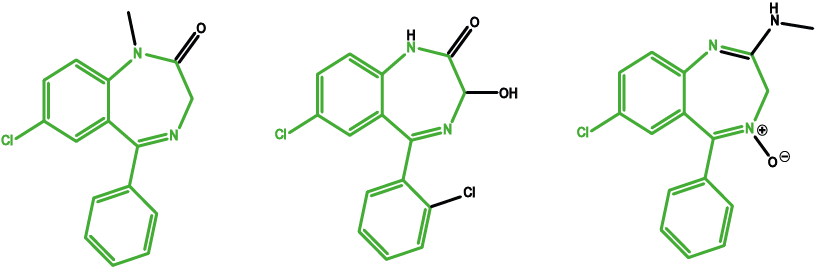
Diazepam is widely used as a prescription tranquilizer (‘rose-tinted spectacles’). Chlordiazepoxide is the most commonly used drug to support alcohol detoxification by reducing EtOH withdrawal effects. Lorazepam is used to treat anxiety disorders and to relief nausea and vomiting during chemotherapy.
Benzodiazepines carry a high potential for dependence to form; this is particularly relevant given how widely this class of drugs has been prescribed to treat anxiety, sleep and convulsive disorders. Tolerance develops quickly, leading to increased doses and withdrawal symptoms following abstinence. As with almost all addictive drugs, the dopamine reward pathway is involved. Dopamine is released due to benzodiazepines interacting with the GABAA receptors (see Figure 5). This positive incentive from the reward pathway mechanism is augmented by negative reinforcement that manifest as withdrawal symptoms such as irritability, anxiety and panic attacks.
Given the undesirable high potential of benzodiazepines to develop dependence, in the UK the so-called Z-drugs (zopiclone, zolpidem) are now the preferred choice of drugs to treat insomnia. The group of Z-drugs interact with the GABAA receptor sites as so-called untypical ligands (their molecular structure is quite different from the benzodiazepine core structure) and it is believed that they are less addictive than benzodiazepines.
Stimulants
Production of content for this website was surely helped by one very widely use stimulant: caffeine.
Between caffeine and cocaine, some may assume that cocaine is the more addictive of these two stimulants. In fact, cocaine use follows more the pattern of a strong habit-forming routine. This may seem a pedantic distinction but many habitually clean their teeth daily without getting addicted to brushing teeth. A strongly established habit should not be trivialised, however: it may be almost as hard to quit a habit as an addiction.
Caffeine on the other hand is known to cause physical dependence with withdrawal symptoms such as headaches and fatigue (there is a joke amongst inpatients in the UK that the quality of caffeinated beverages on wards is so dismal that it accelerates recovery of patients to enable them to find a more palatable solution to their caffeine cravings at home…).
A large class of synthetic stimulants are amphetamine and closely related substances. Amphetamines are powerful stimulants with variable degrees of dependence-forming properties. All amphetamines cause feelings of alertness and being energised. Figure 7 depicts three examples – picked from a very large pool of amphetamine drugs.
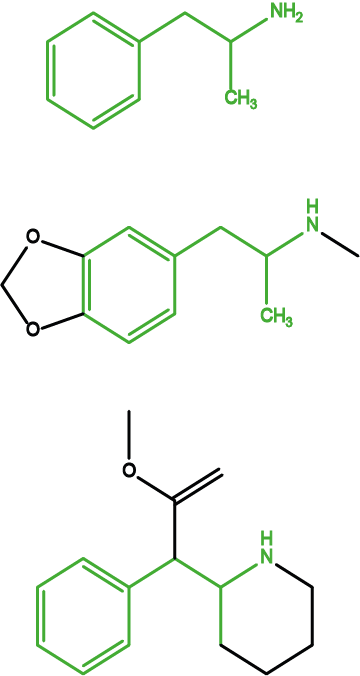
Amphetamine (Figure 7, top) is shown mostly for historical reasons – it represents the core structure of the whole class of these drugs. MDMA (‘ecstasy’) (Figure 7, middle) is a popular recreational drug and mainly gives effects of euphoria. Methylphenidate (Figure 7, bottom) is a clinically used amphetamine drug that is used to treat narcolepsy and hyperactivity disorders such as ADD (attention deficit disorder) and ADHD (attention deficit hyperactivity disorder) and it is debated whether this drug is prescribed too freely and for too long for the latter conditions, especially in children.
Similar to many recreational drugs, most stimulants achieve pleasurable effects through the dopamine-release reward mechanism as well as serotonin release.
Perhaps some extra-motivation to quit excessive drug use when confronted with oral and maxillofacial surgery: there are multiple enhanced risks during and around the time of surgery from all three categories of drugs discussed above, leading to higher morbidity and increased risk of post-operative complications as well as higher risks from general anaesthetics during surgery. Some of these risks are related to various acute withdrawal symptoms (for example, anxiety and tendencies for unsocial behaviour). Continued use leads to constipation and increased risk of respiratory failure (opioids), as well as adverse interactions with other prescribed drugs (all of the drugs discussed above).
Unless you are faced with emergency surgery, there is usually enough time before elective (planned) surgery to engage in a supported round of pre-surgical detoxification. With the right kind of motivation and support in place, this can be done at home.