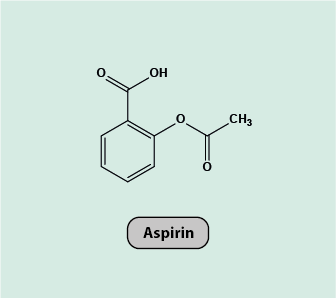
Aspirin
Aspirin, acetylsalicylic acid (Figure 1), is a non-steroidal anti-inflammatory drug (NSAID). It works similarly to other NSAIDs as an analgesic but has additional effects on blood clotting and is a widely prescribed antiplatelet medication, prescribed for example to prevent arterial thrombosis, or as prophylaxis after stroke.
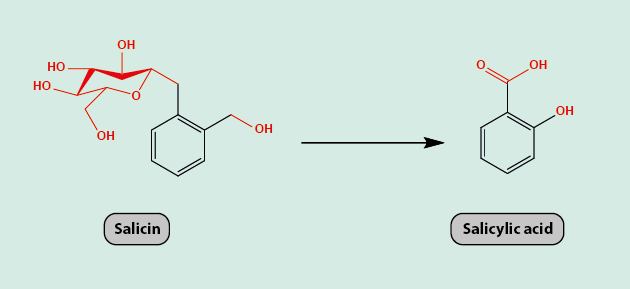
For 3500 years, willow bark was used as an analgesic with the active agent in it being salicin. The metabolism/oxidation of salicin leads to salicylic acid (Figure 2), the precursor of aspirin. Salicylic acid has the same analgesic properties as salicin but causes gastric irritation and so is not tolerated well as a drug.
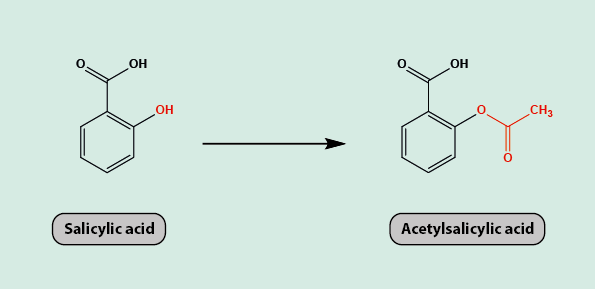
In 1852, Charles Gerhardt, a French chemist, attempted to modify salicylic acid to give acetylsalicylic acid but his product was not stable and hence was not used as a medicinal drug. In the 1890s, a pharmaceutical company began research into making a drug that had the same analgesic action as salicylic acid but lacked the adverse gastrointestinal effects. They managed to modify salicylic acid to give a stable form of acetylsalicylic acid (Figure 3) in 1897 and named it aspirin. Aspirin is a 100 times more potent analgesic than salicylic acid.
Aspirin fell in and out of favour over the years as new analgesic agents with (assumed) fewer side effects, such as paracetamol and ibuprofen, were discovered. Aspirin, however, remains in use, in part due to its antiplatelet effects.
Like other NSAIDs, aspirin inhibits the cyclooxygenase (COX) enzymes. Blocking or inhibiting COX enzymes inhibits the production of prostaglandins and thromboxanes, which are key contributors in the inflammatory response. Thromboxanes promote aggregation of platelets and clotting. Inhibition of their production is thought to produce an analgesic effect.
The COX enzymes insert into the lipid membrane of a cell and form hydrophobic channels to a catalytic site. Aspirin blocks this catalytic site or channel irreversibly which gives long-lasting inhibitory action on the COX enzymes. Aspirin also specifically redirects the metabolic activity of the COX-2 enzyme to the formation of aspirin-triggered so-called lipoxins (short-lived, biochemically active metabolites) with anti-inflammatory effects. One of these lipoxins is known to reduce inflammatory hyperalgesia (increased sensitivity to pain); some lipoxins are thought to be important in the resolution of periodontal disease.
In its function as an antiplatelet agent, aspirin supresses the normal function of platelets also by (long-lasting) inhibition of the COX enzymes. This inhibits thromboxane synthesis for several days, stopping the production of platelet aggregation factors. As the inhibition is long-lasting, the effect on the platelet aggregation factor is over the lifetime of the affected platelet (8 to 9 days). Inhibiting the formation of platelet aggregation factors hinders the normal clotting process.
Aspirin has similar adverse effects as do other NSAIDs, including the risk of gastric ulcers and an increased risk of bleeding in the gastrointestinal tract.