Prostaglandins
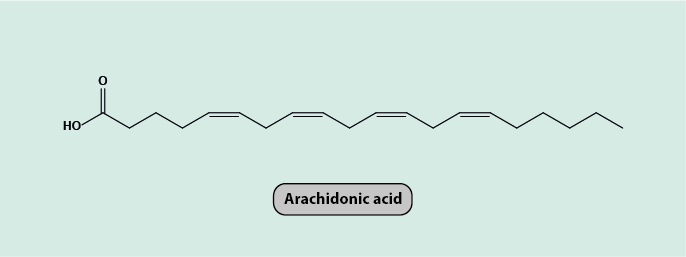
Arachidonic acid is a prototype fatty acid (Figure 1). It is a substance that is mainly taken up from food (meat and eggs) but is also synthesized by the body’s metabolism in humans. From its basic structure, several sub-groups of fatty-acid derivates (lipids) are synthesized in many different cells in the body and are mainly found in the cell membranes. One such subgroup is called eicosanoids, all of which are signalling / messenger molecules in a wide variety of cell and body functions, and which have some hormone-like activities.
Prostaglandins and thromboxanes are all members of the group of eicosanoids. All of these derivatives of arachidonic acid maintain the 20 carbon atoms in the molecular backbone of arachidonic acid, prostaglandins in addition have a five-membered carbon ring in common in their molecular structure (Figure 2).
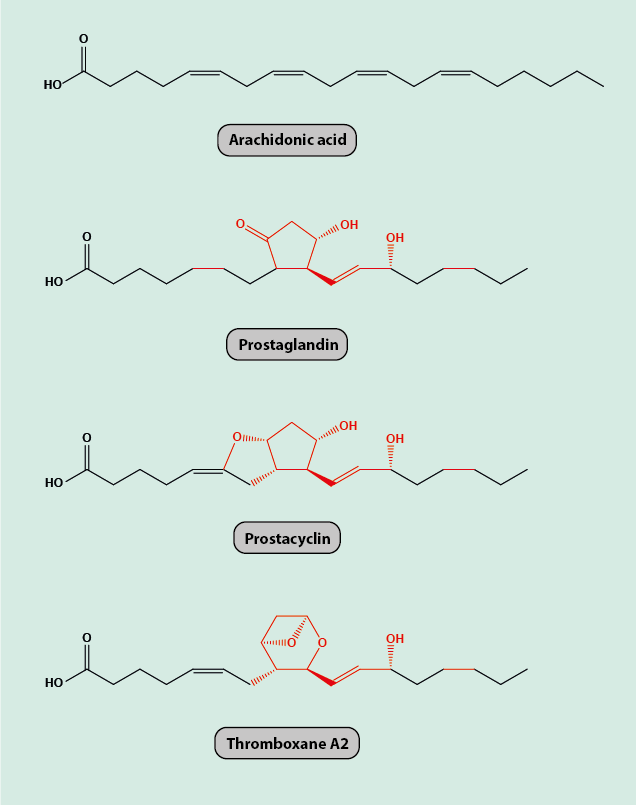
The metabolism of lipids is certainly not a core subject of oral and maxillofacial surgery, but some of the many different physiological roles of prostaglandins and thromboxanes touch on processes that are relevant in a maxillofacial context: pain signalling and sensation and blood clotting. A crude inspection of the biosynthesis of prostaglandins (and thromboxanes) from arachidonic acid actually helps to see these connections.
Prostaglandins are synthesised in the body from arachidonic acid by the enzymes cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). COX-1 is expressed at a constant level in most cells of the body and supports the ongoing synthesis of the various prostaglandins needed for general, normal cell functions. COX-2 is only expressed when cytokines (signalling molecules that are released when there is tissue damage) are present. COX-2 catalyses the synthesis of those prostaglandins needed to deal with tissue damage. These prostaglandins cause painful inflammatory responses. Immunosuppressants, such as glucocorticoids, reduce COX-2 expression. Also, inhibition of the enzymes responsible for the synthesis of prostaglandins potentially has an analgesic effect (for example, aspirin hinders the COX enzymes and thus the conversion of arachidonic acid to prostaglandins).
Most nociceptors (specialised sensors for pain stimuli) in healthy tissue are mechanically insensitive, even to strong stimuli. Prostaglandins can cause these to become excitable by pressure, temperature changes and tissue acidosis (increased acidity of tissue and blood in response to inflammation). This can result in hyperalgesia (excessive pain sensation) due to increased excitability of sensory neurons by a decreased threshold for activation. Some prostaglandins increase pain sensation by reducing the action of inhibitory neurotransmitters (for example, glycine). The overall increased pain sensation resulting from the action of prostaglandins is not due to direct interaction with, and activation of, the nociceptors. Instead, the overall prostaglandin effect on pain sensation is to increase the frequency of pain signals being sent in response to a stimulus.
Prostaglandins are also synthesized in the blood cells and in the cells forming the lining of blood vessels. The involvement of prostaglandins in the inflammatory response in these locations is associated with further local effects. Prostaglandins inhibit the aggregation of platelets (and thus hinder the blood clotting process after injury) and act locally as highly effective vasodilators. Thromboxanes (also synthesized by platelets), are the counterplayers in this mechanism; they are powerful vasoconstrictors and support platelet aggregation. A well-balanced equilibrium between prostaglandins and thromboxanes is one of the essential controls of a normal, healthy blood flow in healthy blood vessels.