Dysplasia
Contents
Oral mucosal lesions are common conditions. Only a small proportion of some of these lesions (especially leukoplakia, erythroplakia, erythroleukoplakia) tend to display dysplastic cells which are considered as potentially premalignant lesions. For the time being, it is not possible to predict if, or how, such lesions may progress to oral malignancies. Current established diagnostic methods are not able to provide such predictive information. This uncertainty makes for difficult decisions about aggressive and invasive treatment options opposed to more conservative ‘wait & watch carefully’ policies, controversially discussed issues. Clearly, this is an area with need for more research: improved knowledge could provide much better diagnostic tools, and in turn reduce unnecessary treatment and ensure treatment is carried out when necessary. With this in mind, below we give a short summary of current methods in histopathology, as well as research into the potential role of biomarkers and genomics in the diagnostic process. Given much research activity on this topic, it is likely that the state of affairs at the time of writing (2020) will change in the not too distant future. We conclude the page with our own interpretation of the current situation.
Histopathology
Taking biopsies of suspect oral lesions is a widely used diagnostic method. However, the method is invasive and requires macroscopic samples to be excised (sometimes repeatedly over time), so there are limits to how often this approach can be taken. In addition, a biopsy sample is just a snapshot of the current state of a tissue and, in the absence of further information, does not provide unambiguous clues as to the likely behaviour of the lesion in the future, nor does it explain why the lesion developed in the first place. On the positive side, a biopsy sample is well suited to classify the degree of abnormal cell growth in tissue (Figure 1) from mild to severe dysplasia.
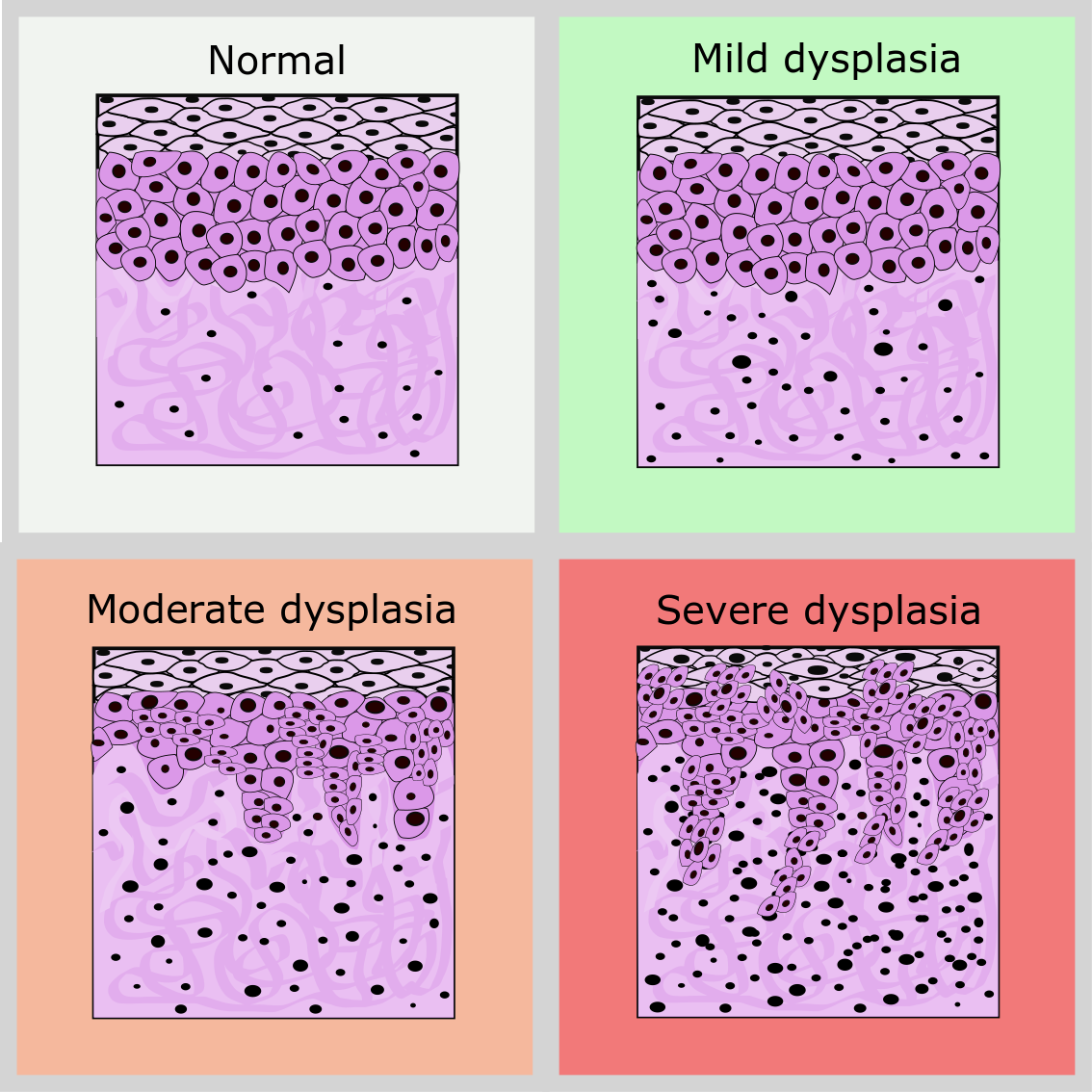
Assessing the density of microvessels, taken as a measure of angiogenic activity, in biopsy samples has been suggested as a measure for potential transition to malignancy. This immunohistological assessment evaluates the membrane glycoprotein CD105 which plays a crucial role in angiogenesis (formation of new blood vessels) and tumour growth. Increasing CD105 levels are identified on going from normal epithelium to mild dysplasia (considered as low risk) to moderate / severe dysplasia (considered high risk). The method has not been established in clinical use: while there are correlations between CD105 levels and severity of dysplasia, the prognostic power of CD105 levels / angiogenic activity regarding the likelihood of progression to malignancy has not been validated.
Biomarkers
Biomarkers are molecules or characteristics present only in certain cell types (such as specific integrins in malignant cells). The use of biomarkers as a method to identify dysplastic tissues with a significant risk of malignant transformation is currently much researched. In addition, biomarkers for certain cell types or biological processes could prove useful in identifying the stage of dysplasia. There are many more potential markers than those we discuss here, the main purpose of this section being to demonstrate the thought process behind developing such diagnostics (and treatments) by using illustrative examples. We divide markers into several categories:
- cell survival and growth (markers involved in the cell cycle, immortalisation and angiogenesis);
- invasion (cell adhesion molecules, matrix metalloproteinases);
- inflammation and signalling (cyclooxygenases & inflammation, EGFR (epithelial growth factor receptor) & signalling pathways).
Cell survival and growth
Cell survival and growth are essential properties for the maintenance of malignancies. For a tumour to grow, cells must divide and proliferate via the cell cycle. The protein cyclin D is a key component of the cell cycle, most notably regulating cyclin-dependent kinases (enzymes). These kinases control the transition into the synthesis stage of the cell cycle by phosphorylating and inhibiting the retinoblastoma tumour suppressor protein, which would otherwise be preventing the cell cycle from progressing. Although linked with invasion and lymph node metastasis, cyclin D is overexpressed relatively equally in the dysplasia grades and oral squamous cell carcinoma, suggesting it could be a marker for dysplastic tissue in general but not in identifying the probability of progression from dysplasia to malignancy. Phosphorylated retinoblastoma protein could be a more effective marker; studies suggest it is more highly expressed in oral squamous cell carcinoma than in premalignant tissues, potentially helping to detect early malignancies. However, the degree of certainty is insufficient regarding clinical use of this marker as a prognostic tool.
To maintain DNA levels and function in new cells, DNA must be replicated before each cell division. However, DNA shortens slightly every time it replicates. Telomeres are protective regions on the end of chromosomes which stop this shortening from removing essential functional genes. In most healthy adult cells, telomerase (the enzyme which synthesises telomeres) is not expressed. In contrast, since malignant cells rapidly divide, telomeres must be replenished (leading to ‘immortalisation’ of cell lines). It is thought that the expression and action of telomerase and its components could be used to detect premalignant cells which need this to maintain DNA length in new cells. 90 % of times where one of these telomerase components (hTERC) was detected in a potentially premalignant lesion, the lesion progressed to malignancy. This observation is potentially significant as a predictive marker as only 5 % of lesions progress to malignancy without hTERC overexpression. Again, this marker has not transferred to clinical use.
As well as the ability to replicate proteins and DNA, growing tumours need an oxygen supply at their centre for cells to survive. The growth factor protein VEGF-A regulates angiogenesis (formation of blood vessels), and its levels in serum appear to correlate with tissue expression levels. Combined with the fact that there appears to be a trend in increased VEGF-A concentrations from oral leukoplakia to oral squamous cell carcinoma, serum VEGF-A levels could be used as a non-invasive diagnostic method (in a ‘liquid biopsy’, a blood sample) to estimate angiogenic potential, and hence risk of malignant transformation (perhaps too much of an assumption as angiogenesis does not equal malignancy). In practise it is not yet certain whether VEGF-A concentration has prognostic value (either in tissue or serum), and thus it is by no means a marker ready for clinical use.
Invasion
Invasion is a key step in malignancy progression where the tumour invades surrounding tissues. Biomolecules which facilitate invasion include cell-adhesion molecules and proteases (enzymes that break down proteins). Detecting and analysing such biomolecules could help detect whether lesions have the potential to become malignant. Reduced expression of cell-adhesion molecules (such as cadherins) between epithelial cells can allow the cells to form a more mesenchymal phenotype. This is part of the epithelial-mesenchymal transition, a conversion of the epithelial cell into a cell with a more migratory, invasive phenotype, facilitating invasion into local tissues and vascular systems (for metastasis). The expression of E-cadherin in particular has been investigated as a potential biomarker, due to its ‘invasion suppressor’ role. Lack of E-cadherin expression may facilitate signalling pathways and invasion which contribute to tumour differentiation and progression, respectively. Studies suggest there is a link between E-cadherin expression and stage of dysplasia / oral squamous cell carcinoma, some even suggesting a continuous decrease until the carcinoma stage is reached. This could make E-cadherin a useful marker, as its levels could then help determine risk of malignant transformation. However, as with VEGF-A (see above), there remains a lot of uncertainty here, and lack of evidence of its functionality in clinical practise.
Matrix metalloproteinases are actively involved in invasion, by degrading the extracellular matrix between the tumour and healthy tissue. Like cell-adhesion molecules, they are also involved in signalling, here promoting malignancy growth by modulating growth factors and cell surface receptors. With oncogenic signalling and invasive action, an increase in matrix metalloproteinases in lesions would suggest potential to develop into malignancies. A study looked at MMP-9 (enzyme matrix metallopeptidase 9) as an example, which was elevated in tissue, serum and saliva of potentially premalignant oral epithelial lesions compared to controls. However, MMP-9 has complex functions, which could be both oncogenic and tumour suppressive. The latter puts into question its ability to act as a biomarker for malignant transformation.
Inflammation and signalling
Inflammation occurs during malignancy development, allowing the recruitment of immune cells to support the tumour, for example tumour associated macrophages. Cyclooxygenases (such as COX-2) are one group of enzymes responsible for inflammation; they catalyse the synthesis of prostaglandins, which increase blood flow and attract white blood cells. For example, via this mechanism COX-2 has an impact on colon, colorectal, lung and breast cancers, and can act as an indicator of reduced survival and treatment response. Due to its role in cancer development, COX-2 has not only been thought of as a potential biomarker, but also a potential target. Thus far, its inhibition has not been successful in malignant lesions, however showed some promise in delaying progress of potentially premalignant lesions, or even removing them entirely. This is thought to be a result of inhibition of the epithelial growth factor receptor (EGFR). EGFR is involved in regulated signalling towards proliferation and differentiation, through a variety of pathways. Its overexpression could therefore be associated with malignancies. Some studies suggest that EGFR expression increases with the severity of dysplasia, and increases further up to the malignant stage. In addition, high EGFR expression in cancer patients correlates with significantly reduced chance of being cancer-free within 10 years, suggesting an important role in maintenance of malignancies. Despite apparent importance in both dysplasia and malignancies, a study on potentially premalignant oral epithelial lesions found EGFR was not associated directly with malignant transformation. As far as head & neck malignancies are concerned, however EGFR provides another example of a marker where its role in progression from premalignancy is insufficiently understood.
Genetics
Genetic analysis could become an alternative diagnostic approach for identification of dysplastic and potentially malignant tissue. In a broad cancer research field, hundreds of genes have been proven or proposed to be involved in malignant transformation and progression. Here we identify some of the genes associated with head and neck malignancies (in particular carcinoma of the oral cavity), especially focusing on genes that could provide early indications on the likelihood of dysplastic tissue to undergo malignant transformation. We also look at wider DNA mutations or abnormalities, including epigenetics (molecules bound to DNA). There is some overlap between genetics and biomarkers; the protein products of some of the genes mentioned could be used as biomarkers, and by extension the genes forming some of the biomarkers could likely be used in genetic analyses. Time will tell which approaches and indicators turn out most useful in clinical practice.
Importance of genetics and the genomic road to invasion
The importance of genetics in progression can be seen by comparing oral lesion progression in smokers and non-smokers. Smoking contributes to common lesion formation in smokers, yet (less common) lesions in non-smokers are much more likely to progress to malignancy. In one study, lesions in non-smokers were found to be twice as likely to progress into a malignancy, and in the case of lesions on the floor of the mouth, there was a 38-fold increase in likelihood of progression for non-smoker lesions. This, of course, does not at all advertise smoking as healthy or helpful, it only emphasises the importance of genetics in malignant transformation. Where lesions are more likely to be genetically caused (in non-smokers), the progression rate is much higher. A specific example of an important gene in malignant transformation is TP53, the gene encoding the p53 tumour suppressor protein. In both dysplastic and carcinoma tissue, changes in TP53 are the most frequent genomic mutation, and it is widely thought of as an important gene in oral cancer development. This is unsurprising when considering the function of its product, p53. Dubbed the ‘guardian of the genome’, the p53 protein is activated by DNA damage and protects tissues from this damage being replicated and escalated in the next cell generations. It does so either by arresting the cell cycle and allowing DNA repair, or through inducing apoptosis (programmed cell death) to kill cells where damage is too great to fix. When TP53 is mutated or deleted (which is the case for over 50 % of malignancies), mutations can more easily accumulate, and hallmarks of malignancy are enabled such as replicative immortality (as the cell cycle is not stopped anymore by damaged DNA) and cell death resistance (p53-mediated apoptosis prevented). Mutated p53 has even been proposed to have oncogenic functions itself, with single amino acid changes in its structure allowing new interactions, for example with other transcription factors (such as MLL1, a fusion protein with an essential role in human leukaemia).
Genes altered in dysplasia, and in malignancies
The differences between normal and dysplastic tissue, as well as dysplastic and tumour tissue, can to an extent be explained by genetic changes. One study found 47 genes altered in oral leukoplakia with dysplasia, compared to leukoplakia without dysplasia. The most notable pattern was in downregulated genes, which were often associated with organisation and signalling pathways involving the extracellular matrix. This suggests that the development of a reactive stroma (supportive tissue of epithelial tissue) could be an early occurrence in dysplastic development. The extracellular matrix is not the only difference between normal and dysplastic tissue. RNA sequencing revealed a difference in cells present, with an apparent increase in numbers of cytotoxic effector immune cells (such as mast cells) present in the dysplastic tissue. This likely reflects the immune system being activated in dysplastic tissue, aiming to remove abnormal cells (in similar fashion to immunosurveillance of malignancies). In contrast, from dysplasia to malignancy, there is a decrease in effector immune cells, and a sharp increase in inflammatory cells (such as a doubling of macrophages). This likely marks the recruitment of immune cells to the malignancy, forming tumour-associated macrophages and neutrophils which contribute to the immunosuppressive function of the tumour microenvironment. Hence a change in immune cells (marked by a change of gene expression) could potentially provide clues to the stage and likelihood of progression.
Other genes associated with malignant transformation from dysplasia include genes involved in binding and building of cytoskeletal proteins (such as actin, an important protein for cell movement and internal organisation), genes involved in formation of apical/adherent junctions (organisation of the layers of epithelium), and HOX genes (involved in controlling overall body shape and development). Changes to cytoskeletal protein binding and formation, as well as loss of apical junctions (which form links between epithelial cells), together suggest a role of invasive phenotype formation in malignant progression. HOX genes are broadly involved in tissue architecture maintenance, but in a malignancy context could potentially be involved in angiogenesis, proliferation, invasion, metastasis and many more related processes. Although upregulated mostly in the normal to dysplastic transition, HOX gene upregulation in dysplastic tissue appears to be associated with progression, with fewer lesions without HOX upregulation leading to malignant transformation.
Genetics & biomarkers
Genetic-related changes which could also be used in a biomarker context include DNA ploidy status (number of sets of chromosomes in a cell), loss of heterozygosity (cells containing two (or more) different alleles of a gene), DNA damage response and epigenetic (non-genetic modifications to DNA, such as binding of proteins) events. DNA ploidy status refers to the number of chromosomes present in a cell, and DNA aneuploidy is the term used to describe differences in chromosome number compared to regular cells. Studies have suggested DNA aneuploidy, a sign of genetic instability, is more common in the latter stages of dysplasia, and may cause increased likelihood of malignant transformation whether in normal or dysplastic tissue. Loss of heterozygosity is thought to contribute to malignancy development through the loss of the single functional allele of an important malignancy-related gene (such as TP53; see above). The occurrence of multiple key losses of heterozygosity (including genes for both p53 and p21 proteins) was found to significantly increase risk of malignant progression. However, progression of lesions at different sites in the mouth, and unexplained heterogeneity between lesions, bring these findings into uncertainty.
DNA damage response is a process which detects and repairs DNA damage. Molecules involved in this response could be used as biomarkers, as some of them have been found to peak in dysplasia (when immune response is occurring), then dip following progression to oral squamous cell carcinoma. An example is phosphorylated H2AX (a protein involved in recruiting repair proteins and arresting the cell cycle), which follows this trend. Finally, some epigenetic changes could be used as biomarkers for malignant progression. Methylation is an example (chemical modification of a protein molecule by incorporation of (a) CH3, methyl, group(s)). Methylation silences genes, preventing their transcription. For example, a tumour suppressor protein (p16) is hypermethylated in around 40 % of oral dysplasia cases and 80 % of head and neck squamous cell carcinomas – so could be an indicator of likely progression.
Summary
There remains an urgent need for improved prognosis in dysplasia and malignancy diagnostics. The current best practise of histopathology is invasive and while it is the standard for diagnosis of malignancy, fails to predict future progression of dysplasia, whilst studies on novel biomarkers and genetic clues for progression remain largely at the theoretical stage. Biomarkers and genetic mapping demonstrate various associations with progression to malignancies but lack any clear-cut prognostic powers. This is demonstrated by the few illustrative examples of the thinking behind these suggested markers (there are many more), none of which have any proven ability to act as prognostic indicators.
To us, this suggests that, although the thought process behind many ideas is sound and studies have been carried out carefully, perhaps the overall strategy is flawed – simply because the wealth of information accumulated to date has not made progress to provide any identifiable clear pathway. Unpredictability is an inherent feature of malignancies (and, in fact, of all living organisms) – their mutations, metastases and recurrences are what make them such difficult diseases to treat. Thus, it makes sense to suggest an alternative paradigm that there is no simple, single, quantifiable marker to predict progression from dysplasia to malignancy. In addition, there must be further limitations to the current studies – otherwise surely some of markers (especially those claimed to increase with the stage of dysplasia and further increase following malignant transformation), would have progressed into clinical practise as prognostic tools.
The problem may lie in the generally accepted theory of ‘field cancerisation’. This is the linear model for cancer development which was developed in the early 1950s:
- Normal cells mutate to contain some of the features required for malignancy.
- Mutated cell lines expand (as have properties which enable their survival over other cells), resulting in a cancer-primed population (or ‘field’) of mutated cells. Cancerised fields (such as dysplasia) contain mutations pushing towards cancer, but are not yet malignant.
- Cells in the cancerised field eventually develop into a malignant tumour.
If this theory is correct, it suggests cancerised fields of cells (which could be detectable) will be present in every developing malignancy, and development is an orderly and predictable process. Thus, informed by this model, much research (as discussed above) has been devoted to finding (a) specific marker(s) for this pre-malignant state. However, this linear model is almost certainly too simplistic, and fails to account for the heterogeneity of malignancies and the unpredictability of malignant progression.
Deviation from this linear thinking could encourage search for more effective, alternative clues for progression of malignancies. It is interesting to note that a similar situation exists regarding the low response rates to immunotherapies and the current inability to predict who may or may not benefit from immunotherapies and/or may suffer serious adverse effects. Recent critical reviews about the state of affairs with immunotherapies also conclude that the search for the one marker may well be in vain, for fundamental reasons. Some preliminary genomic mapping of the clonality of recurrences and metastases in head & neck malignancies paints a similarly confusing picture of a multifactorial situation, with little indication that a simple (and prognostic) picture may emerge from simply amassing more data.
At his point in time, an epilogue to this chapter seems in place. We could opt for a rather blunt statement in saying that so far biomarkers and/or genetics and/or histopathology all share some elements of the usefulness as prognostic tools reminiscent of the usefulness of a chocolate teapot. Alternatively, we could borrow a quotation from the epilogue of Brecht’s play ‘The Good Person of Szechwan’:
“Wir stehen selbst enttäuscht und sehn betroffen
Den
Vorhang zu und alle Fragen offen.”
The play is an illustration of the impossibility / difficulty of being a good person in an evil world; the play does not offer a conclusive ending. In the epilogue of the play an actor addresses the audience, acknowledging that a nasty open end was slipped upon the audience (“the curtain closed & all questions open”), and that they are invited to not only draw their own conclusions but also to “write the happy ending of the play!” themselves.