Integrins
Integrins are a family of proteins that form a component of cell walls and generally fulfil a diverse range of roles in the cellular biochemistry and metabolism. We discuss this group of proteins because of their potential for real-time assessment of surgical margins in maxillofacial oncological surgery, a particularly important aspect of head and neck surgery. We give a brief overview of structure and function of integrins, how they interact with / bind to other molecules, and how their specific properties can be used to visually distinguish malignant cells from healthy tissue. We conclude by comparing the potential of integrins for the assessment of surgical margins with current methods (mostly a special kind of biopsy, a frozen section) and some other currently explored techniques, such as Raman spectroscopy or mass spectrometry.
Structure and function of integrins
Integrins are a family of proteins located in the outer surface of cells, the cell membrane. They belong to a category of proteins known as transmembrane proteins. They play an important role in connecting cells to each other and to the region surrounding cells, the extracellular matrix. There are different types of integrins, with slightly different structures and, therefore, slightly different properties. The general structure of integrins is shown in Figure 1.
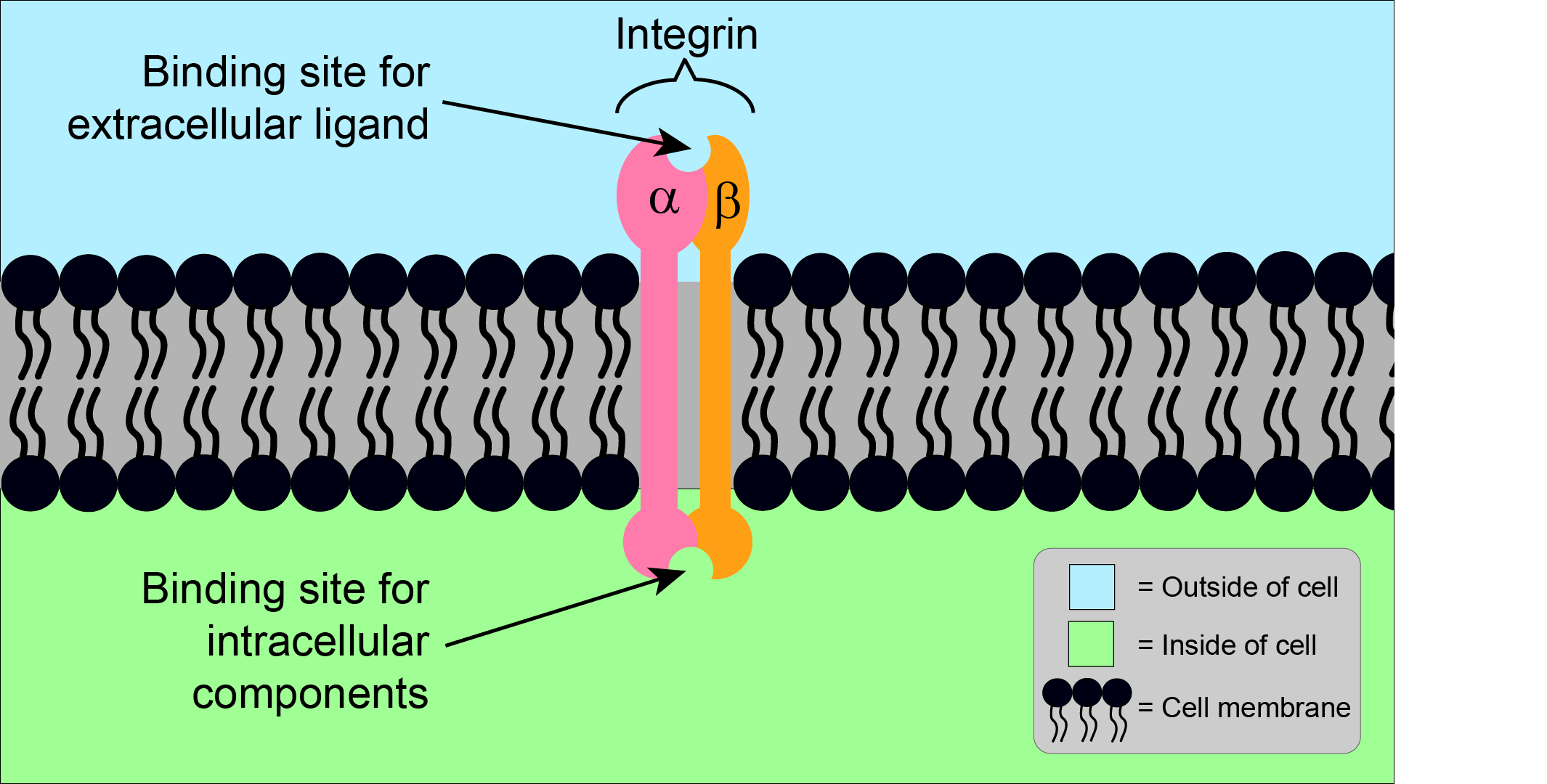
Generally, integrins consist of one alpha (α) subunit, and one beta (β) subunit. With these two different subunits, integrins are referred to as a heterodimer. Humans have 18 different α subunits, and 8 different β subunits, which together can form at least 24 different heterodimers. Each of these integrins has a distinct structure and function, including the ability to bind to different components (ligands) of the extracellular matrix. One particular feature of ligand - integrin binding is a common motif of three particular amino acids (arginine, glycine, aspartic acid; the ‘RGD sequence’), which is located on many ligands. Some integrins can bind to multiple ligands, and some ligands can bind to multiple integrins.
Integrins are involved in cell - cell and intra-cell - extracellular matrix binding. Integrins not only help join the cell and its extracellular space physically, but also play an important role in communication between them, through signalling in both directions (Figure 2 and Figure 3).
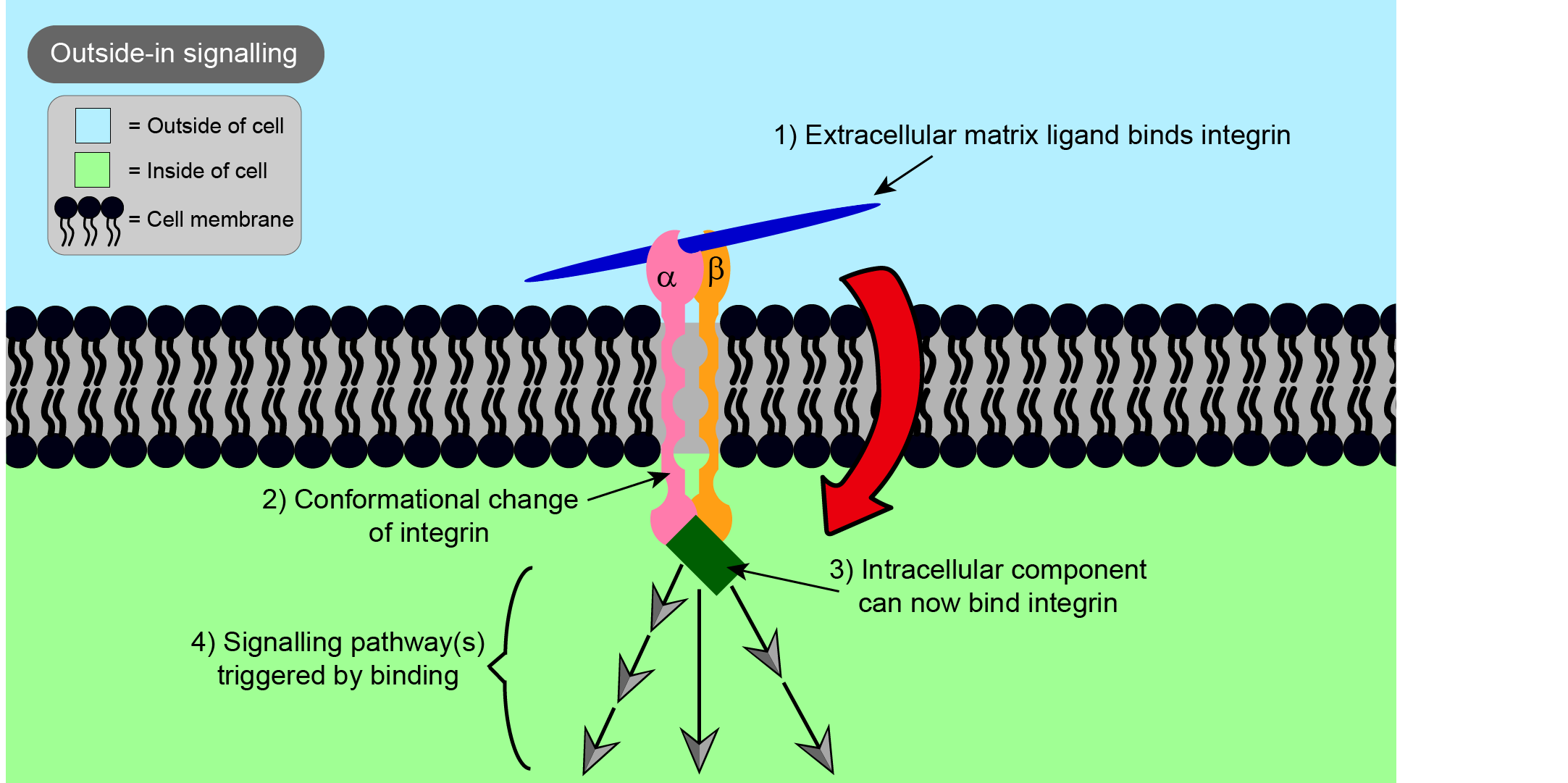
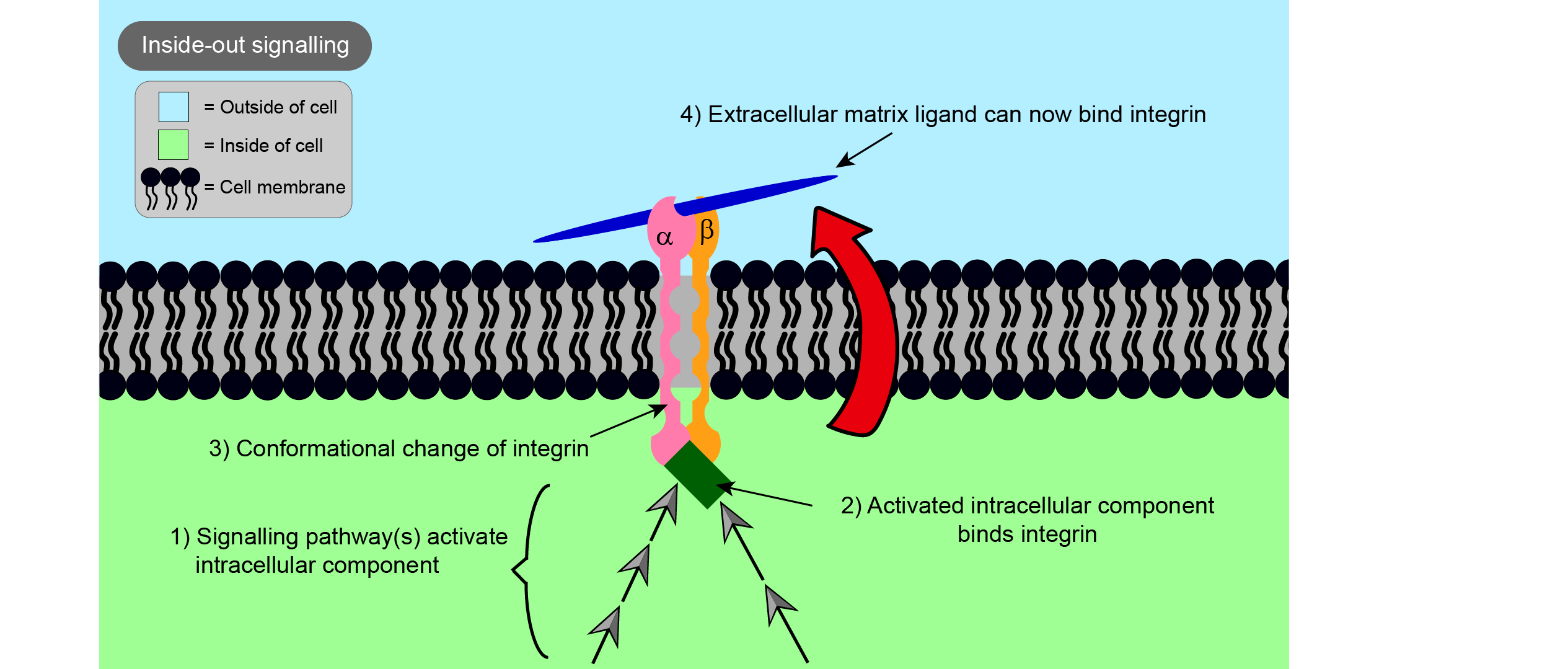
‘Outside-in’ signalling is the term used to describe signals starting from the extracellular matrix, whereas ‘inside-out’ signalling is when signals start from the inside of the cell. In each case, binding of a ligand to one end of the integrin causes a change in the overall shape of the integrin, in this way passing on a message to the other side of the cell membrane. In the case of outside-in signalling, this can impact on important processes such as cell proliferation (the rate at which cells divide), cell survival, and cell movement. The survival of many types of cells is reliant on binding to the extracellular matrix, so integrins are important in preventing programmed cell death known as apoptosis, a part of the regular renewal processes of a living organism.
Integrins in healthy and in malignant cells
Integrins are thought to be involved in many conditions, including malignancies, some viral infections (such as the Epstein-Barr virus), and some autoimmune diseases (such as Sjögren’s syndrome). The specific type of integrins involved in diseases are often found at elevated levels in damaged cells and tissue. For example, certain integrins involved in malignancies are found in much higher levels in tumour cells than in surrounding healthy cells. Thus, in principle, specific integrins may offer a means to distinguish malignant cells from healthy tissues. In particular, the integrin αvβ6 has been identified to be present at high concentrations in the invasive front of malignant oral tumours (squamous cell carcinoma, the most common oral malignancy) but is only present at very low levels in normal adult cells in healthy tissue. The human eye cannot see individual cells or protein molecules, so some form of visual marking of the high concentration of the diagnostic integrin is needed (Figure 4).
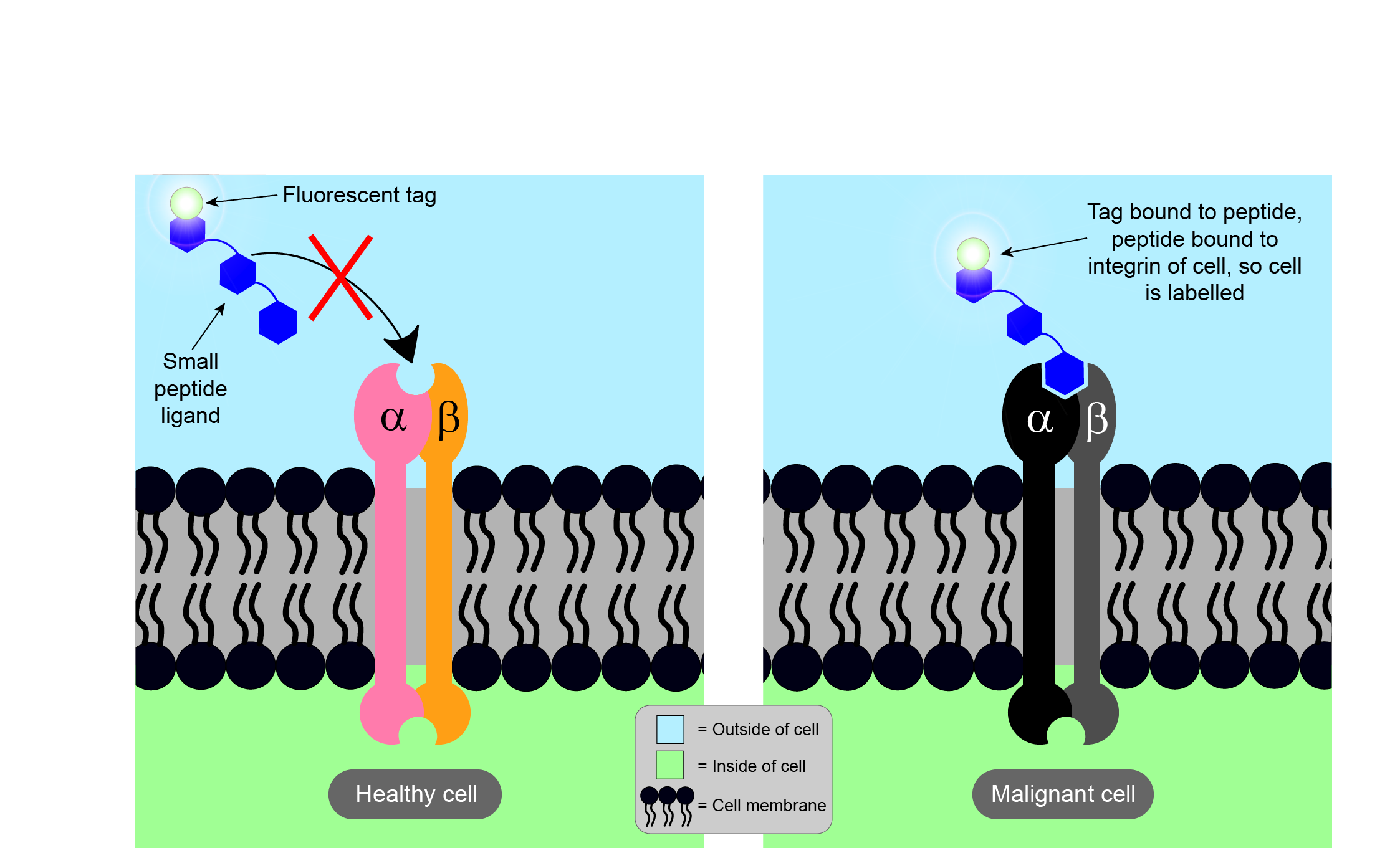
The way to achieve visualisation of the diagnostically relevant integrin is by binding a specific (small) peptide to it. As it is known that integrin αvβ6 binds to RGD peptide sequences (see above), small peptides containing this sequence and binding solely to integrin αvβ6 have been identified; attaching a fluorescent label to the marker peptide then makes malignant cells visible when inspected with the appropriate light and/or fluorescence microscope. In a nutshell, this process effectively paints malignant cells in a different colour to healthy tissue.
The ability to label malignant cells and easily distinguish them from healthy tissue has an obvious application as a tool to assess surgical margins in the operating theatre (see below). Such a ‘designer peptide’ which solely binds the specific integrin enriched in a particular tumour could also be used to guide toxic substances to malignant cells, whilst sparing healthy cells from exposure. Similarly, such integrin – ligand interactions could be used to interfere with malignant cells more directly, affecting their movement (relevant in the development of metastases), their proliferation and their signalling, and possibly inducing the selective apoptosis of malignant cells.
Integrins in the evaluation of surgical margins in maxillofacial surgery
Current methods to assess surgical head & neck tumour margins include taking frozen sections of tissue, or the assessment of cytological samples where hard tissue needs to be evaluated. These forms of biopsies rely on speedy access to / action of the pathology laboratory and are known to suffer from some inaccuracies. The human eye and tactile information, in conjunction with imaging results (CT and MRI) obtained before surgery are the remaining tools.
Achieving a clear margin in oncological surgery is not just important in maxillofacial surgery but is highly relevant for all areas of tumour surgery. It is highly important to have such margin information available intraoperatively, rather than having to wait for regular histology / pathology results (which may take several days). Accordingly, many efforts are underway to find, test and establish new ways to assess surgical margins in the theatre. For example, breast surgery may benefit from using mass spectrometry for this purpose, and Raman spectroscopy is also explored as a new real-time in-theatre assessment technique. Both methods hold promise (and are being explored for applications in maxillofacial surgery), but both methods depend critically on the availability of suitable data bases for comparison with a ‘fingerprint’ of the actual specimen. One may thus assume that these two methods will be most successful for tumour surgery where there is little tissue heterogeneity (little variation of tumour cell types) between cases and a meaningful data base for comparison can be assembled in a straightforward manner. Unfortunately, this is not the case for head & neck malignancies which represent a diverse and heterogenous group of malignant tumours. In addition, in the head and neck region the requirement for evaluation of surgical margins is particularly challenging as often complex defects are created, and there is a delicate balance between achieving a clear enough margin without harming vital functionalities or without unnecessarily causing other functional impairments.
The potential of integrins for evaluation of surgical margins in head and neck malignancies has been demonstrated clinically for the integrin αvβ6, including the evaluation of bony margins. The method remains to be fully evaluated. There is a clear potential advantage to the integrin approach for head and neck malignancies over other currently explored technologies: the method does not depend on comparisons with a database, it directly addresses the case at hand and thus is well placed to deal with heterogenous groups of tumours.