Inflammation
Contents
Having explored the normal working of the immune system and the resulting inflammatory responses in the context of infection and wound healing, there can be little doubt that a system as sophisticated as this body defence system will be error prone. Small deviations from perfect function may have major effects on many different body functions.
Malfunctions of the immune system
Dysregulation of the immune system and the resulting malfunctioning inflammatory responses include an underactive immune system (hypoactive), an overactive immune system (hyperactive), or aberrant inflammatory responses of the immune system attacking the host’s own cells (autoimmune conditions) after misreading the self-cells as foreign / invaders. There is often considerable overlap between a hyperactive immune system and autoimmune conditions.
In a maxillofacial context, typically only a few such immune-system related conditions are encountered (see below). These conditions could either be genuine maxillofacial conditions, or other systemic conditions with signs and symptoms that will be addressed in a maxillofacial clinic.
Hypoactive immune system conditions include
- suppressed immune system after organ transplants (to prevent rejection of a transplant by the immune system of the host which would normally recognize and attack the presence of non-self cells; a suppressed immune system leads to generally increased infection risk)
- chronic and advanced renal (kidney) disease as well as the treatment of these conditions (dialysis) are associated with considerable dysregulation of the immune system (with a complicated simultaneous presence of up-regulated (chronic inflammation) and down-regulated subsections of the immune system; generally increased infection risk)
- long-term treated HIV infection leads to similar damage and infection risk from dysregulation of the immune system
- deficiency of a particular antibody, IgE, as revealed by blood tests is associated with an increased risk for fungal infections such as by candida albicans; low (or absent) concentration of IgE in the blood may be caused by genetic disorders.
Hyperactive immune system conditions include
- a wide range of (common) allergic reactions of varied severity and duration, caused by all kinds of normally harmless substances (allergens) in the environment (such as pollen, foods, certain metals and metal ions, or medications). There is a common underlying mechanism to all these different reactions, which can range from mild irritations to sometimes severe or life-threatening (anaphylactic shock) allergic reactions. IgE antibodies bind to some surface receptor of the allergen, and thus initiate a misguided, sometimes violent, inflammatory response. Blood tests for allergies typically show elevated levels of IgE antibodies. Repeated and prolonged exposure to an allergen over time may enhance the severity of the inflammatory / allergic response because the immune system carries on learning and in some instances misunderstanding, about its roles and tasks.
- orofacial granulomatosis, prolonged and repeated swellings in the orofacial area, in particular the lips, is known to be an inflammatory process related to overreactions of T cells (subtype of white blood cells involved in the immune system), occasionally it is the symptom of an allergy.
Autoimmune conditions include
- recurrent mouth ulcers , especially more severe cases
- arthritis affecting the jaw joint (a rare variant of arthritis which more commonly affects other joints)
- type 1 diabetes, where the underlying autoimmune reaction destroys the insulin-producing cells and permanently alters and dysregulates the immune system in general, leading to a generally increased risk of infections
- Sjögren’s syndrome is an autoimmune condition which affects the salivary glands and causes problems with dry mouth (xerostomia) and dry eyes
- bullous pemphigoid and pemphigus vulgaris are autoimmune conditions which cause blistering of the skin and oral mucosa (lining of the mouth) and mainly affect the elderly.
Similar to its role in driving hyperactive immune system behaviour in general (see above), the antibody IgE is also known to contribute to misguided inflammatory responses in autoimmune reactions, for example targeting self-cells in joints in arthritis.
Medications which interfere with the immune system responses
Medications to down-regulate or block the immune system response
There are three main categories of medications in this category: antihistamines, corticosteroids, and more recently anti-TNF biologics.
Antihistamines in some ways counteract the effects of histamine and are mostly known for their role in treating allergies. Histamine is a small molecule (see Figure 1) with many different roles in the body’s metabolism.
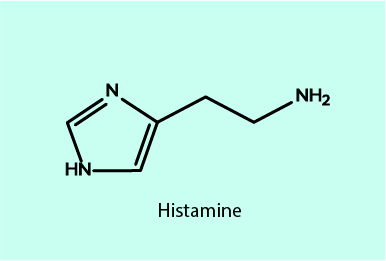
Histamine is involved, amongst further roles, in the inflammatory response and immune system regulation in general, it promotes the secretion of gastric acid, and in the brain, it acts as a neurotransmitter in the regulation of the sleep-wake cycle by maintaining the awake / alert state. It may be surprising that such a small molecule has so many different roles, but it is precisely because it is a small, slightly polar molecule that histamine can fulfill these many roles: it can be easily dispensed everywhere in the body, it can bind to many different receptors and structures, and it is easy to generate in the cell cycle as and when needed.
Histamine is synthesised in specialized white blood cells (basophils) and mast cells. Throughout the body these cells are particularly common in regions with potential need to respond to injury or infection: blood vessels, nose, mouth, generally all inner and outer body surfaces. In the brain and in the stomach other, non-mast cells generate histamine.
Histamine is released in response to an antigen by mast cells and basophils when the chain of events is triggered by IgE antibodies on these cell surfaces binding to an antigen / allergen. If the response is to an allergen, this is known as an allergic immune response. Histamine works in similar ways to cytokines in the inflammatory response by causing vasodilation (the cause of common itching and swelling in an allergic reaction).
Most antihistamines designed to reduce the effects and symptoms caused by histamine release do so by blocking the histamine receptors (called histamine-1; see below) so that the allergic reaction stops at that stage. This is schematically illustrated in Figure 2.
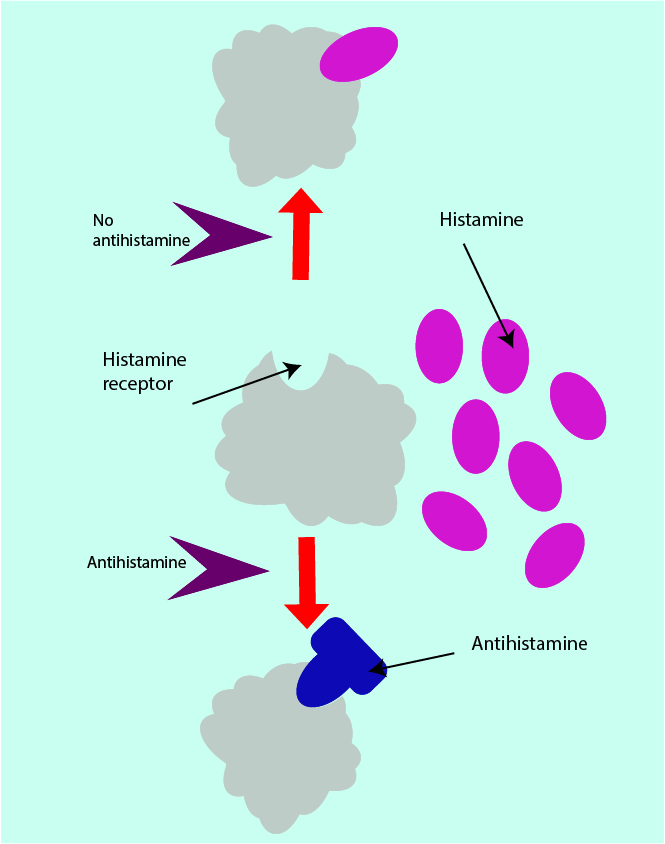
With the histamine receptor site blocked, also the clinical signs and symptoms of an allergic reaction are blocked, or at least reduced. However, the mast cells and basophils continue to generate and release histamine as long as the allergen is not removed.
There is a large number of substances with antihistamine properties, interacting with and hindering the histamine-1 receptor sites. Acrivastine, diphenhydramine and doxylamine (see Figure 3) are long-established such antihistamines.
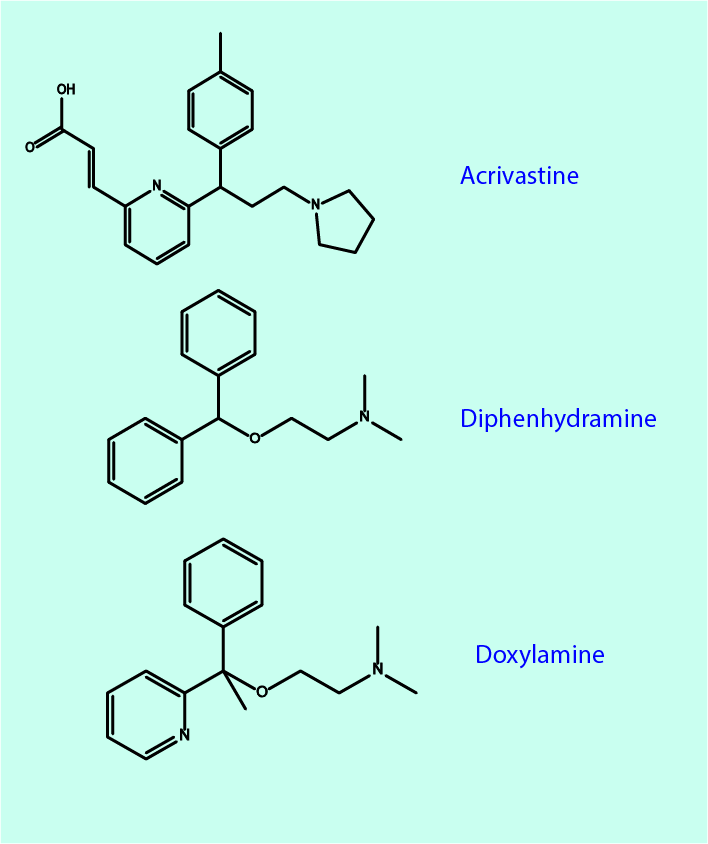
They all share a pattern of prominent unwanted effects, such as causing dry mouth and drowsiness. The mechanisms leading to drowsiness are readily explained: most antihistamines easily pass the blood-brain barrier. Since the role of histamine in the brain is to support alertness and prevent sleep to take over, blocking or hindering histamine action in the brain has the obvious consequence of leading to drowsiness and fatigue. In fact, some substances with antihistamine properties can be used as sedatives. Newer antihistamines are designed such that they should less easily cross the blood-brain barrier and thus cause less drowsiness.
A slightly different type of histamine receptor (called histamine-2) is found on the stomach cells responsible for secreting gastric acid and activated by histamine. For example, ranitidine, a well-known medication to treat indigestion (heartburn), is an antihistamine that blocks the histamine-2 receptor site and thus reduces the secretion of gastric acid. This can be exploited to either treat gastritis (inflammation of the lining of the stomach), or as a protective agent to prevent gastritis from developing as a consequence of long(er) term use of oral analgesia with NSAIDs (nonsteroidal anti-inflammatory drugs); see below.
Corticosteroids are hormones, naturally produced in the adrenal glands (a pair of small glands situated above the kidneys). Synthetic corticosteroids are modelled closely upon those occurring naturally. There are two types of corticosteroids: at their natural, very low concentration glucocorticoids predominantly have an effect on the metabolism of proteins and carbohydrates, whereas mineral corticosteroids regulate the balance of water and electrolytes (salts) in the body. Mineral corticosteroids can interfere with the production of histamine (see above) by white blood cells and so reduce the amount of histamine released in the allergic inflammatory response. Figure 4 shows the molecular structures of a few (of very many) selected corticosteroids.
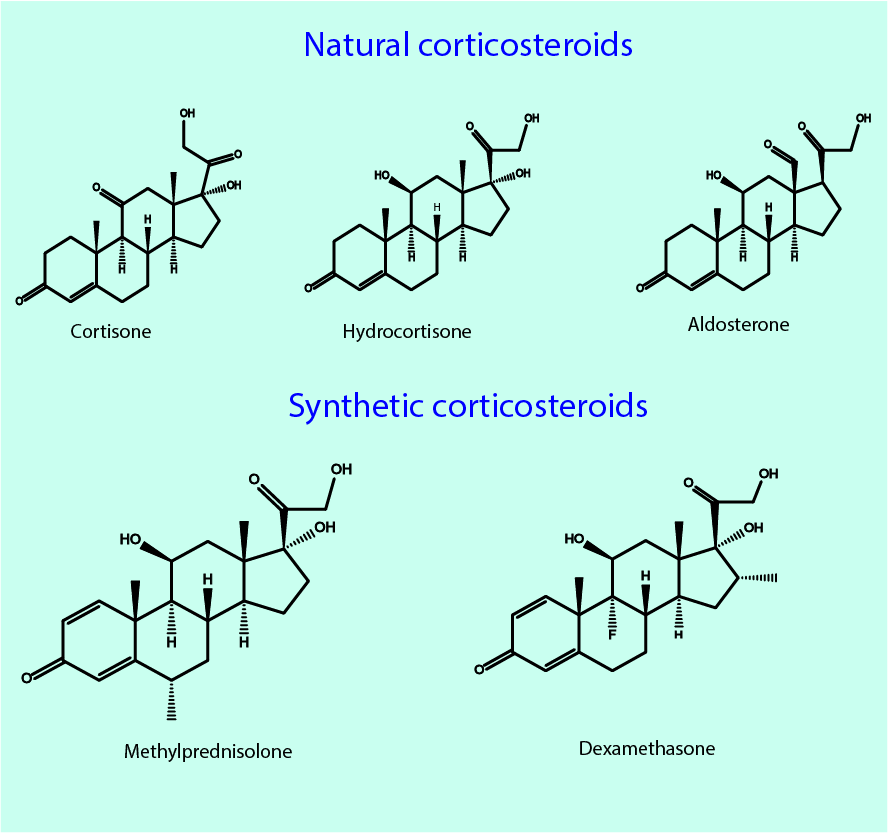
Cortisone, hydrocortisone and aldosterone are produced in the adrenal glands in very small quantities. Aldosterone mainly regulates electrolytes and water metabolism. Cortisone and hydrocortisone are glucocorticoids. The bottom part of Figure 4 shows just two examples of very many synthetic corticosteroids, highlighting the close chemical similarity between natural and synthetic steroids.
At concentrations exceeding the natural very low levels, glucocorticoids have anti-inflammatory and immunosuppressant properties, as well as their impact on metabolism. The anti-inflammatory properties of glucocorticoids were discovered in the 1940s. Figure 5 schematically illustrates the mechanism of action of glucocorticoids.
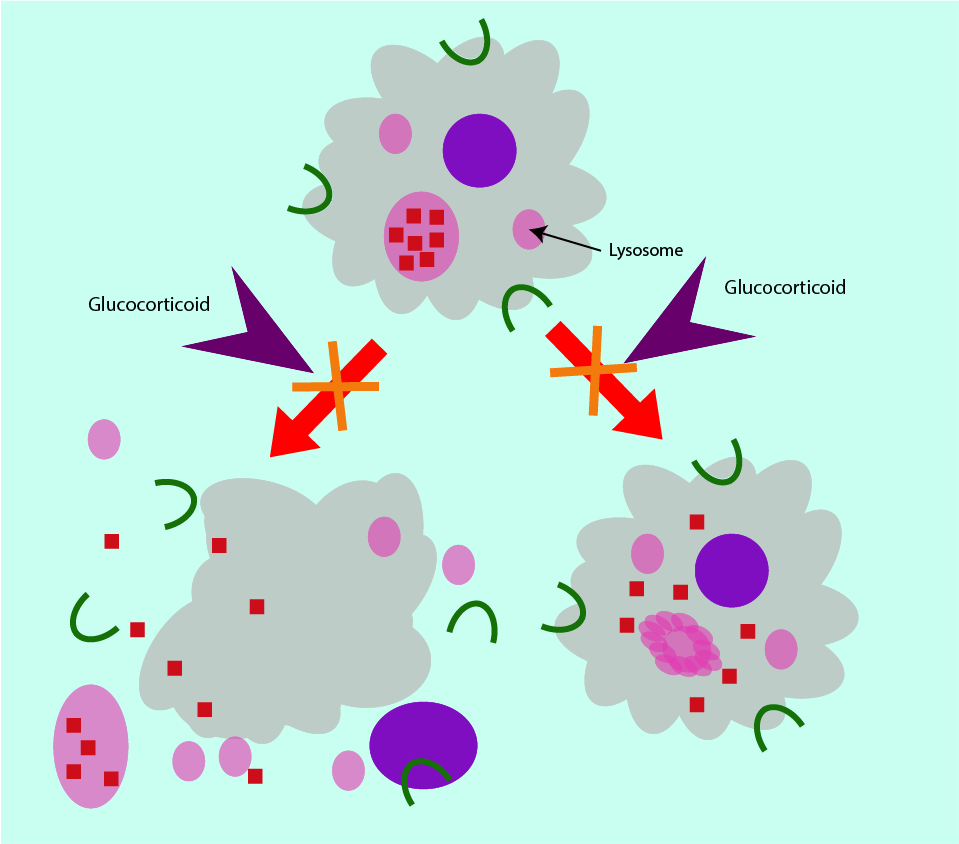
At the cellular level as part of their anti-inflammatory properties, glucocorticoids stabilise the membranes of lysosomes. This prevents the release of aggressive hydrolytic enzymes into the cytoplasm of the cell and ensures continued normal function of the cell. In addition, glucocorticoids also stabilise the outer membranes of the various types of white blood cells, preventing rupture of cells. Glucocorticoids regulate the permeability of capillaries so that an overactive immune response is curbed because white blood cells remain contained in the blood vessels. Glucocorticoids further prevent phagocytes and leukocytes (types of white blood cells which kill and digest pathogens) from accumulating. By inhibiting phagocytosis (the engulfment of pathogens) as well as decreasing the amount of antibody formation (in response to the presence of a pathogen) the immune response is reduced.
Corticosteroids are mainly used as anti-inflammatory agents, often in the treatment of autoimmune conditions, but are also used as immunosuppressants (for example to prevent organ rejection after organ transplants). They can be administered in many different ways, topically, orally or intravenously.
The active, mainly short-term use of a small range of corticosteroids (such as hydrocortisone, methylprednisolone and dexamethasone; see Figure 4) in maxillofacial surgery is to suppress swelling after elective surgery, sometimes in severe infections where swelling around the airway may be an issue (although some corticosteroids can reduce the effectiveness of some antibacterial agents ) and sometimes for a putative improvement of microvascular flow.
The long-term use of corticosteroids (as prescribed for a range of conditions outside a maxillofacial clinic, for example severe allergic reactions, rheumatoid arthritis, colitis, asthma, lupus, psoriasis) is more of an issue and requires careful consideration of benefits and risks as these medications have many unwanted effects, including serious side effects. The list of unwanted effects includes hypertension (high blood pressure), oedema (fluid retention), muscle weakness, osteoporosis, electrolyte imbalances, weight gain and fat deposits, thinning of the skin and slow wound healing, increased risk of infections. When people are on long-term medication with corticosteroids for some non-maxillofacial related condition(s), this can still have impact on a range of considerations in maxillofacial surgery, from fitness for surgery, to risk assessment for increased risk of infection from immunosuppression, to the general effects of steroids on metabolism (including slow wound healing), This is the case because corticosteroids overall suppress the the hypothalamopituitary axis. This axis includes the hypothalamus, the pituitary gland and the adrenal glands, which together form one of the four main regulatory metabolic and endocrine regulation systems in the body.
NSAIDs (nonsteroidal anti-inflammatory drugs) have both anti-inflammatory and pain-relieving properties, with the latter being their main effect and the former a welcome added bonus. Well-known (over the counter) analgesics such as ibuprofen, diclofenac or aspirin belong to this category of widely used pain-control medications.
Nearly 50 years ago, a protein with signaling properties to initiate tumour necrosis was discovered and named tumour necrosis factor, TNF. Soon TNF was known to be involved in multiple regulatory cell cycles, in particular it is now known to be the master of pro-inflammatory cytokines. This inflammation-promoting role of TNF is also critical in autoimmune inflammatory conditions. Unsurprisingly, research was soon devoted to block this biological role of TNF for the treatment of autoimmune inflammatory conditions such as rheumatoid arthritis. Such biological agents were termed anti-TNF biologics, and five such substances were licensed for treatment of inflammatory conditions in 2017 (infliximab, etanercept, adalimumab, golimumab and certolizumab pegol), with more candidates in various stages of development, from laboratory phase to pre-clinical and clinical studies.
The strong anti-inflammatory effects of blocking TNF by adalimumab and similar agents are undoubtedly wonderful for some people afflicted by painful inflammatory conditions which can be very effectively treated with these agents. However, only half of people with such conditions respond to treatment with anti-TNF agents. In addition, anti-TNF agents are a double-edged sword in that their clinical effectiveness in reducing the inflammatory response is closely coupled with their powerful immunosuppressant properties (given the multiple cell regulatory roles of TNF), leading to a number of potentially serious adverse effects. Such unwanted effects include a much-increased risk of infection as well as increased risks of development and/or recurrence of malignancies.
The exact role and mechanisms of TNF and anti-TNF agents warrant much more research. For example, anti-TNF therapy leads to worsening in almost all cases of multiple sclerosis, and anti-TNF therapy can trigger new autoimmune conditions (for example, psoriasis). Immunotherapies in general, in the treatment of malignancies as well as of inflammatory conditions require a far better understanding of their fundamental biology.
Medications to up-regulate the immune system response
Such medications are not really used for, or particularly relevant to maxillofacial conditions specifically.
As a matter of completeness, we just mention briefly that in general, however, there are roles for such medications. In particular the treatment and prevention of viral infections such as HIV or variants of hepatitis, may make a boost to the body’s immune response desirable. There is a wide range of such biological immunostimulant agents (interferons) available for therapeutic use. Another immunostimulant can be histamine (see above) which is used for this purpose in the form of histamine dihydrochloride.