Reconstruction
Before discussing the various types and locations of defects and options for their reconstruction in maxillofacial surgery, we examine the various types of grafts and flaps most commonly used.
Grafts
A tissue graft involves complete separation from the donor site and subsequent transfer to the recipient site.
A graft can consist of
- skin
- mucosa
- fascia
- bone
- cartilage
- nerve.
Skin grafts
Skin grafts can be harvested as
- split-thickness grafts (all of the epidermis (outer layer of skin) and part of the dermis (tissue layer beneath epidermis)) – the donor site will heal / resurface by epidermal cell migration (Figure 1);
- full-thickness grafts (the epidermis and dermis layers) – the donor site needs to be closed because no epidermal structure remains to resurface, and if left, the donor site will heal slowly with a contracted scar.
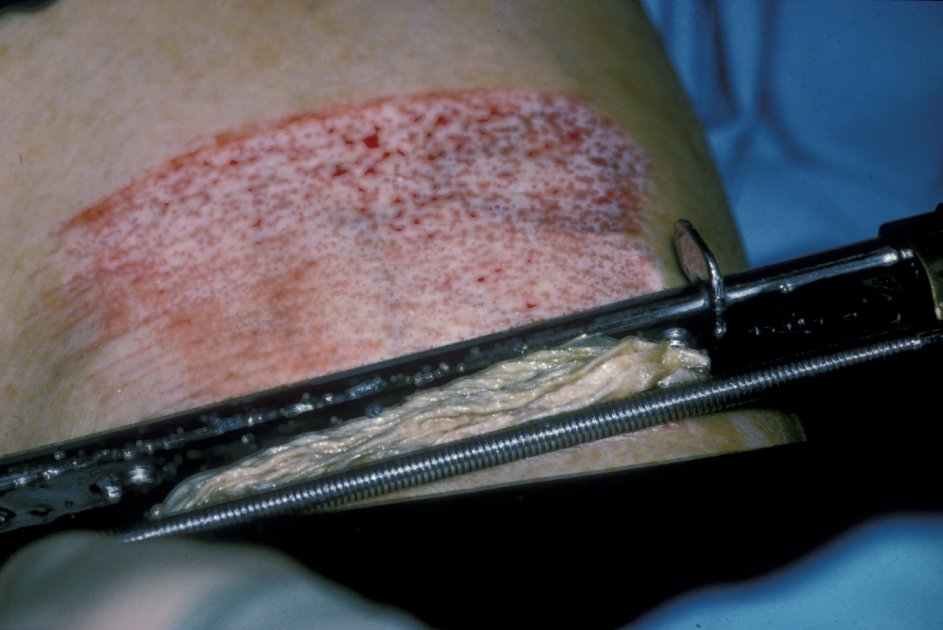
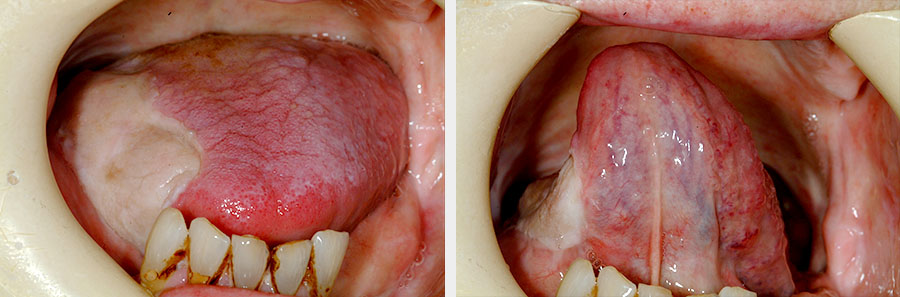
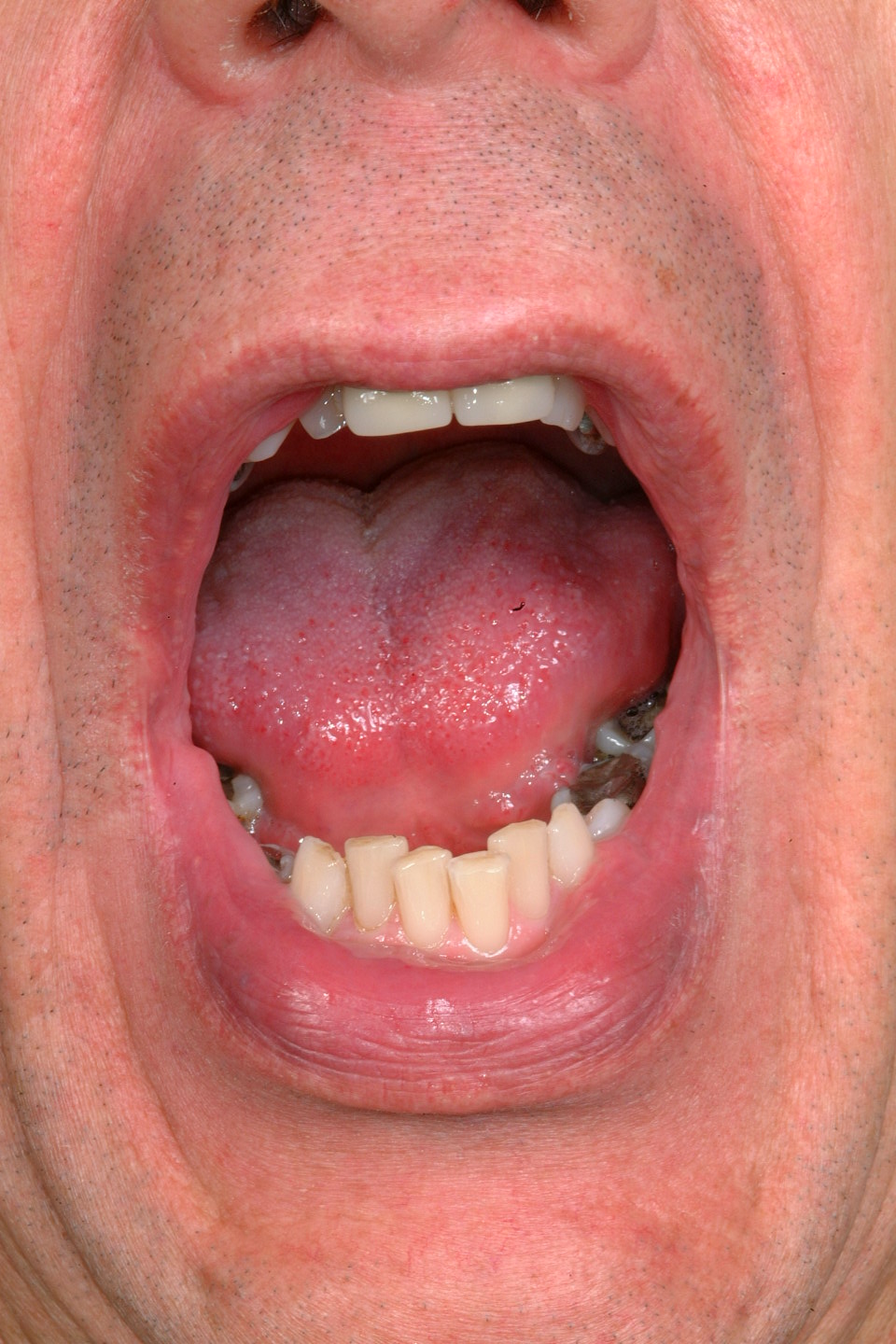
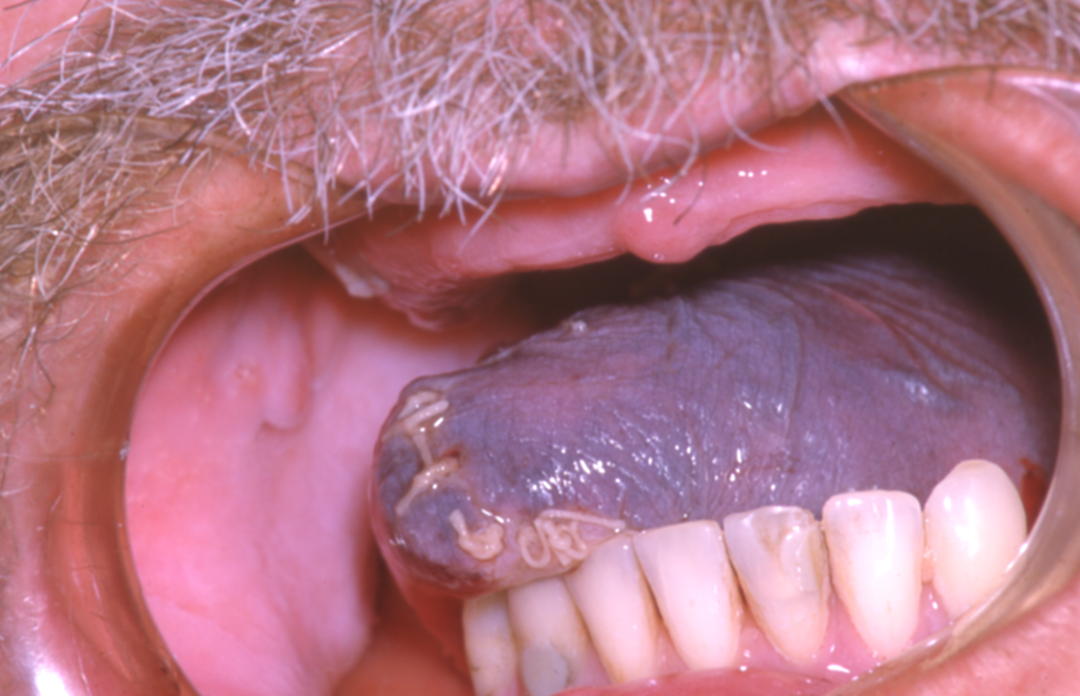
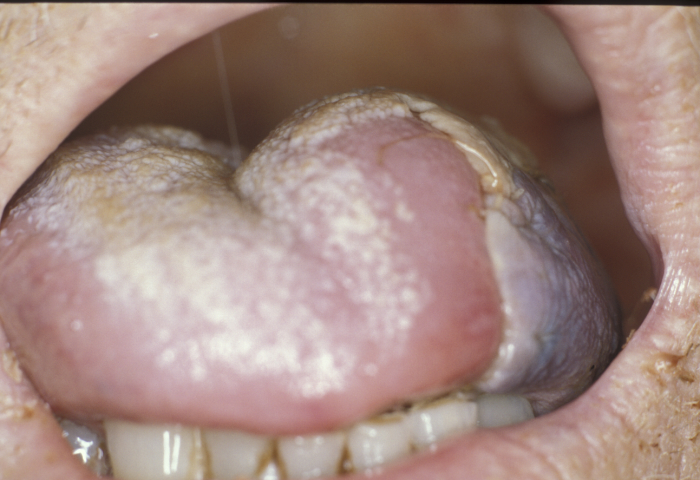
Skin grafting is a simple and popular soft tissue reconstructive technique. It requires a healthy bed to take. The donor site is dictated by factors such as size of graft required, appearance of donor site scar and harvested tissue, presence of absence of hair, access during surgery and mobility of the patient after surgery. Split-thickness grafts can provide viable reconstruction of the tongue in some cases (Figure 2).
Split-thickness donor sites include the upper and lower limbs as well as the abdomen. Grafts can be harvested manually with a specialised knife (adjustable for thickness of graft) or an electric dermatome. The donor site requires dressing changes over a period of weeks; more pain can be experienced at the donor site than at the recipient site. The graft can also be paler in appearance (Figure 3).
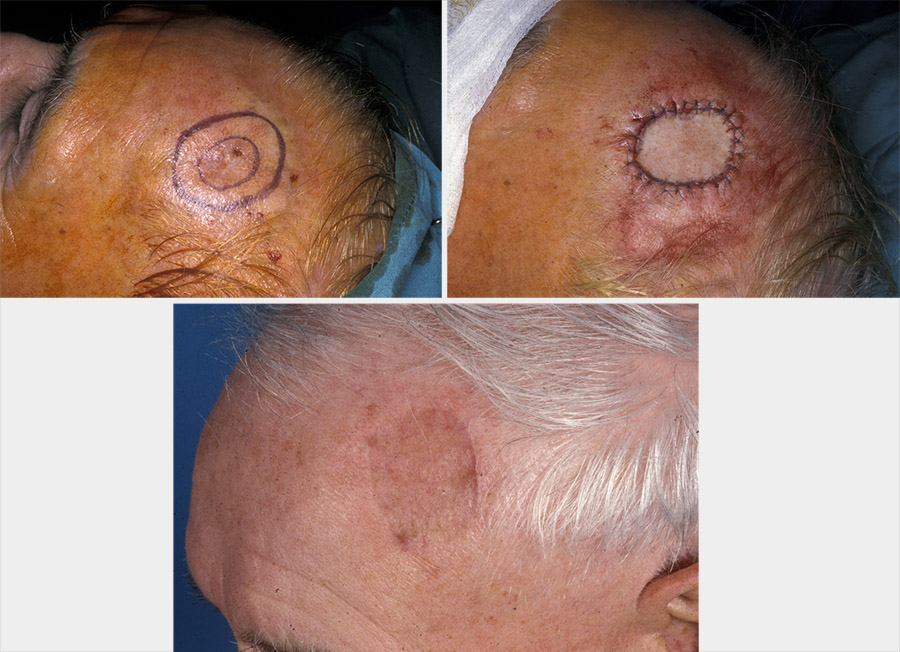
Full thickness grafts for the face are usually harvested from the face (preauricular, postauricular, upper eyelid or nasolabial fold), neck or abdomen. The choice of donor site depends on the colour match, the size required and the patient’s and surgeon’s preferences. The donor site is primarily closed, leaving a linear scar preferably in an inconspicuous site.
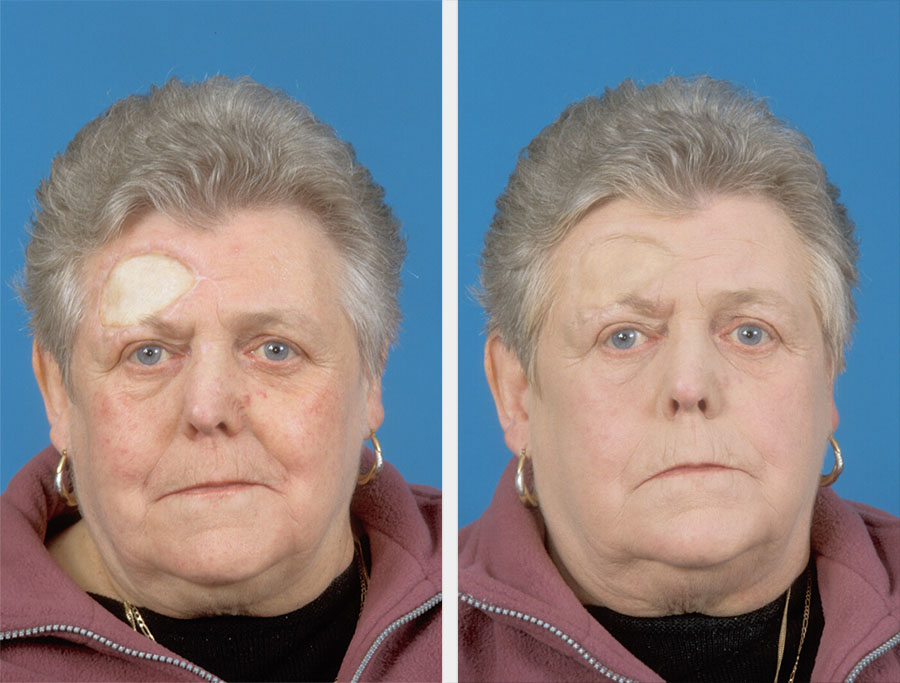
Both split-thickness and full-thickness skin grafts are useful in repairing superficial oral and facial defects. The full-thickness skin graft is particularly effective in nasal, eyelid and forehead defects (Figure 4).
Split-thickness skin grafts can be meshed (perforated with holes and stretched to increase the graft size) to cover large defects. In some instances (for example, surplus skin graft, or infected recipient site), placement of the skin graft can be delayed for up to three weeks provided the harvested skin graft is stored at 4°C and moistened with saline on paraffin gauze.
Split-thickness skin grafts take more readily (thinner tissue requires less vascular ingrowth) but has a poorer outcome (tendency to contract, with a thinner characterless look) than full-thickness skin grafts (Figure 5). The success rate is generally high. Failure is usually the result of mobility of the graft, haematoma or infection.

Mucosal grafts
These grafts can be harvested from the buccal mucosa (cheek) with primary closure, or the palatal mucosa (will re-epithelialize). The advantages of using a mucosal graft to reconstruct oral defects are a good tissue match and the ease of harvesting in the same operative field. However, it introduces a scar in the same area and may further impair function. Mucosal grafts are useful for gingival (gum) or alveolar repairs, and as inner lining in eyelid repairs.
Fascial grafts
The fascia is a strong band of connective tissue. It can be harvested from the temporalis fascia or from the fascia lata in the thigh (Figure 6).
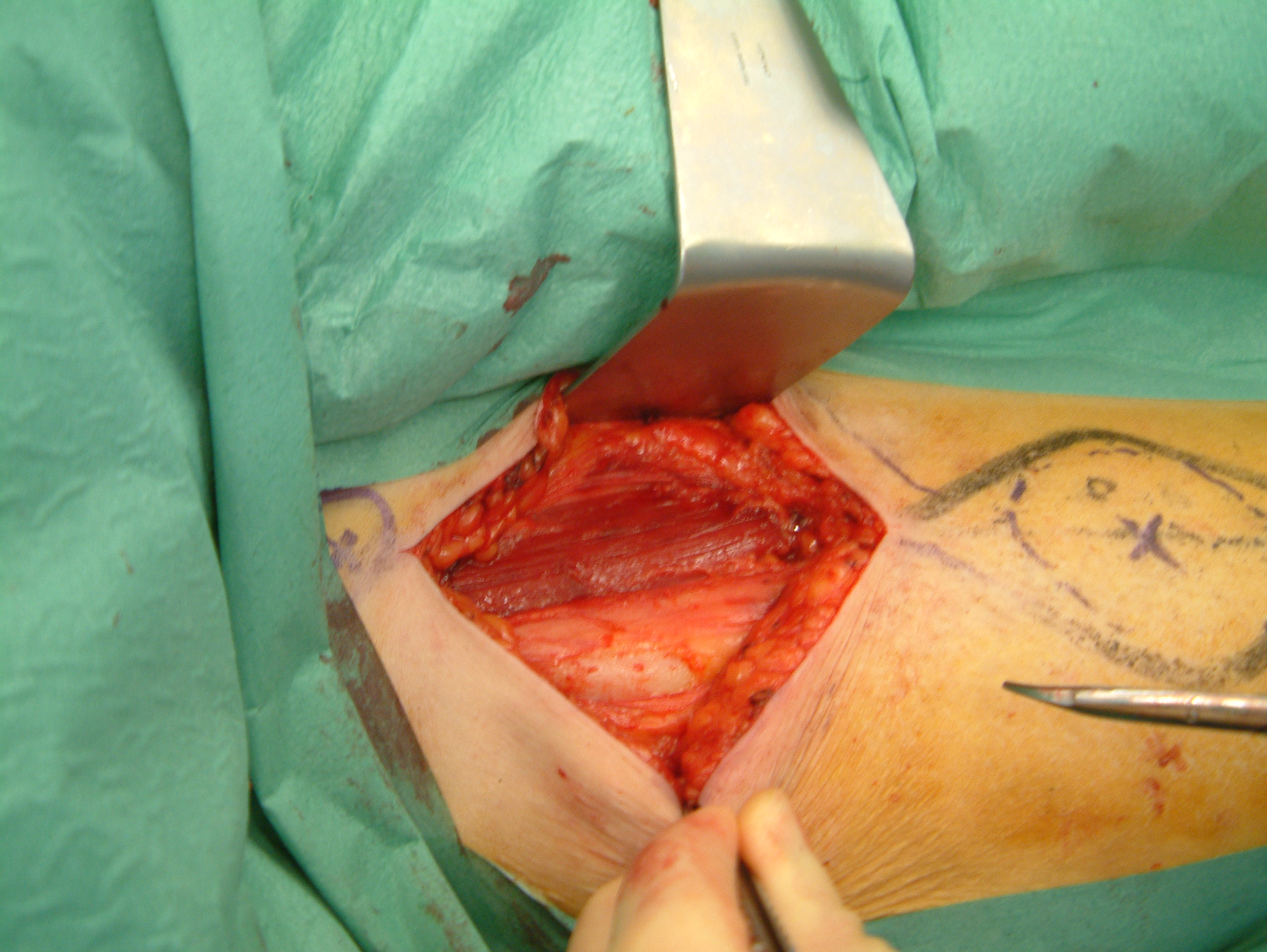
Uses in the head and neck region include dural repair in craniofacial procedures and as facial slings (as part of static facial reanimation in the treatment of a paralysed face. A fascial graft will contract and scar considerably if it is used in the mouth or on the skin surface.
Bone grafts
Bone grafts can be:
- cancellous (medullary (middle section) component, which is mushy but rich in bone-forming components;
- cortical (hard structure useful for supporting role);
- corticocancellous block (Figure 7).
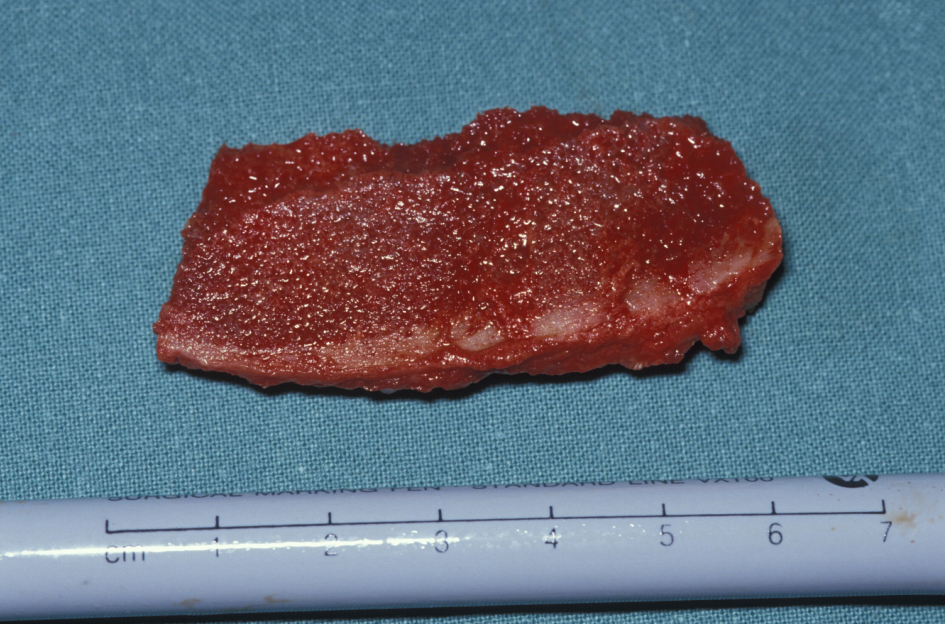
Bone grafting is an established and relatively straightforward hard tissue reconstructive technique. A bone graft is used to provide a rigid structure (form, contour and mechanical support) and osteogenic (bone growth) potential (Figure 8). Osteogenic potential occurs by providing a scaffolding structure (osteoconduction role), which is later replaced by the ingrowth of new bone. This active process is thought to take place using the grafted bone’s osteogenic / osteoinductive potential, in particular the bone morphogenic proteins (group of proteins that initiate bone formation and activate the bone-forming cells (osteoblasts)). There is much active research ongoing in the fields of bone tissue engineering, bone synthesis, prelamination, bone growth factors.
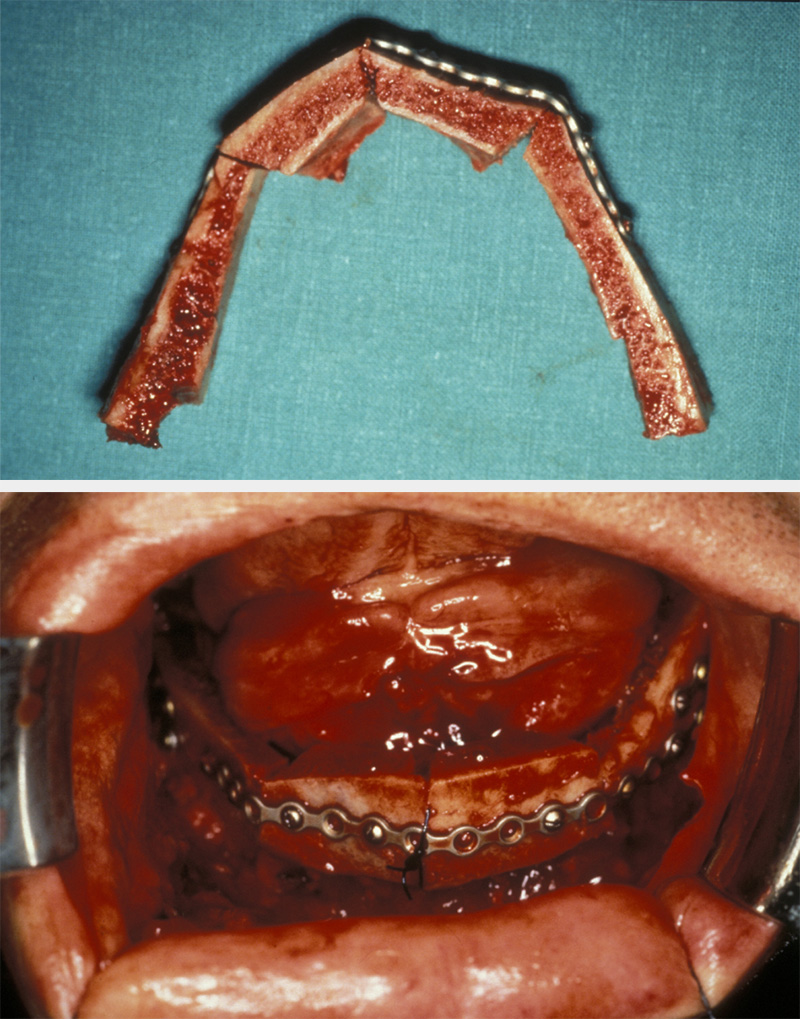
Bone grafts can be used to fill bone cavities, augment bone deficient areas or replace and reconstruct bony loss (see Figure 8). Cancellous bone grafts are commonly used in secondary cleft repair and they provide osteogenic potential in treating nonunion of fractures in conjunction with rigid fixation (and are less risky for unwanted effects than the use of systemic growth factors. Platelet reduced plasma (produced at time of operation by centrifuge) is an increasingly popular alternative. Rigid bone grafts are often used to provide continuity and support in jaw defects and to replace orbital volume in trauma.
Common donor sites for nonvascularised bone graft harvesting are the iliac crest (top part of pelvic bone / hip; provides cancellous and cortical bone), tibia (shinbone; cancellous), ribs, calvaria (skull) and mandible.
There are various commercially available synthetic bone substitutes (for example, demineralised bone matrix, calcium carbonate (CaCO3), calcium hydroxyl apatite (Ca10(PO4)6(OH)2, a synthetic version of the natural bone mineral; Figure 9) which can add bulk and some scaffolding to the native bone but are seldom useful entirely on their own. There is one exception to this: synthetic grafts heal well in calvarial (skull) graft sites. Cadaveric bone has not found favour in mainstream maxillofacial reconstructive surgery. A short, low-morbidity, operation to acquire somebody’s own bone from a suitable and regenerable source is usually best.
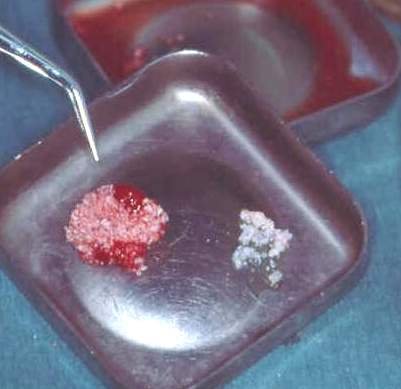
Bone grafting is generally successful. The requirements for successful bone grafting include a healthy bed (to enable vascularization), relative immobility (for bony union) and freedom from infection. An autogenous bone graft is initially devoid of blood supply and it revascularizes by ingrowth of capillaries from the adjacent bed. The existing bone ‘scaffold’ is eventually replaced by its resorption and the deposition of new bone. Cortical bone tends to take longer (but can provide useful mechanical support during this time) than cancellous bone. Cancellous bone can be incorporated into the host skeleton within three months.
Other than donor site morbidity (for example, with iliac crest harvest, scarring, numbness on side of leg and groin, aching and deformity), the main adverse event is bone graft loss. The presence of infection is a contraindication to free bone grafting because the success rate is dramatically reduced. Tissue beds after previous radiotherapy or with adjuvant radiotherapy planned / ongoing are commonly associated with bone graft loss. In these instances, vascularised bone transfers (see below) have a substantially higher success rate.
Cartilage crafts
Cartilage grafts are useful in specific areas of reconstruction, particularly for the nose. The best source are autogenous grafts. Three donor sites are commonly used: the nasal septum, the ear and the ribs. Cartilage has a low rate of rejection and can provide good support for overlying soft tissue. It has little or no antigenicity (does not trigger a strong inflammatory response by the adaptive immune system) and can be harvested as a composite graft incorporating thick skin or mucosa.
Nerve grafts
Nerve reconstructions are usually carried out as free cable grafts, although nerve transfers can be incorporated as part of a vascularized free tissue transfer (see below). The cable nerve graft gains its blood supply from the tissue bed and provides the scaffold for reinnervation. Common applications for nerve grafts in the head and neck region are facial nerve reconstruction following radical parotid gland surgery and reinnervation of the mental nerve following mandibular resection with loss of the inferior alveolar nerve. Nerve grafting requires microsurgical re-anastomosis under magnification.
Common donor sites for head and neck reconstruction include the great auricular nerve (runs in front of the ear) and the sural nerve (sensory neve in the lower leg and foot). The superficial branches of the radial nerve (upper arm) have been used as part of vascularized tissue transfer techniques (see below). Although often deemed technically successful, the reinnervation generally comprises some 30 % of a complete recovery. It is worthwhile in facial nerve reconstruction because there will often be better facial tone, even if complete reanimation is not achieved. Success using cadaveric reconstituted nerve grafting has been reported, and this may obviate the donor site arguments against nerve grafting.
Flaps
A flap is tissue that is transferred with its own blood supply from the donor site to the recipient site. Therefore, a flap is much less dependent on the recipient site for take. The use of flaps with their own blood supply allows more tissue bulk and predictable healing and viability (Figure 10).
There are two main patterns of blood supply for flaps; random and axial. A random pattern flap relies on a general subcutaneous blood flow. As there is no specific vascular supply (no named blood vessel), there is a limit to the amount of tissue transferable before distal flap necrosis occurs. In random pattern flaps this is usually a ratio of 1 : 2 for base to length of the flap. In the head and neck, due to its rich vascular network, a ratio of 1 : 3, and sometimes more, is safe.
An axial flap is based on a specific vascular pedicle running in the subcutaneous plane. It supplies an area of overlying skin (the vascular territory is termed the angiosome). The vascular supply of an axial pattern flap is more reliable than that of a random pattern one. Provided the vascular pedicle is safely included, much larger and longer flaps can be raised.
Myocutaneous flaps (involving muscle and skin) such as the pectoralis major or latissimus dorsi flaps (see below) are usually a combination of the two patterns of blood supply: an axial muscular supply with a random patterned supply to the overlying skin by small ‘perforating’ vessels. The skin paddle is dependent on small musculocutaneous vessels arising from the vascular pedicle(s) deep to the muscle. The axial blood supply means that muscle-only flaps can also be raised. Myocutaneous flaps tend to be bulky although the denervated muscle atrophies (shrinks) with time.
Classification of flaps
There are two main categories of flaps: pedicled flaps and free flaps.
Pedicled flaps can be
- local flaps (for example, tongue flap, buccal fat pad, nasolabial or submental flaps; see below)
- distant flaps (for example, deltopectoral (axial pattern) or pectoralis major (myocutaneous) flaps; see below).
Free (microvascular tissue transfer) flaps are distant flaps, with common donor sites including
- radial forearm
- fibula
- deep circumflex iliac artery (DCIA)
- anterolateral thigh
- rectus abdominis.
Pedicled flaps
Local flaps are a popular reconstructive method for small defects, particularly when the wound bed is not suitable for grafting (for example, for a through-and-through defect, or if only a cortical bone surface is available) or the wound is too big for primary closure. Local flaps use adjacent tissue which has the ideal characteristics required for the defect. The blood supply depends on random patterned subdermal plexus of vessels. The rich vascular supply of the face and oral cavity is well suited to this technique.
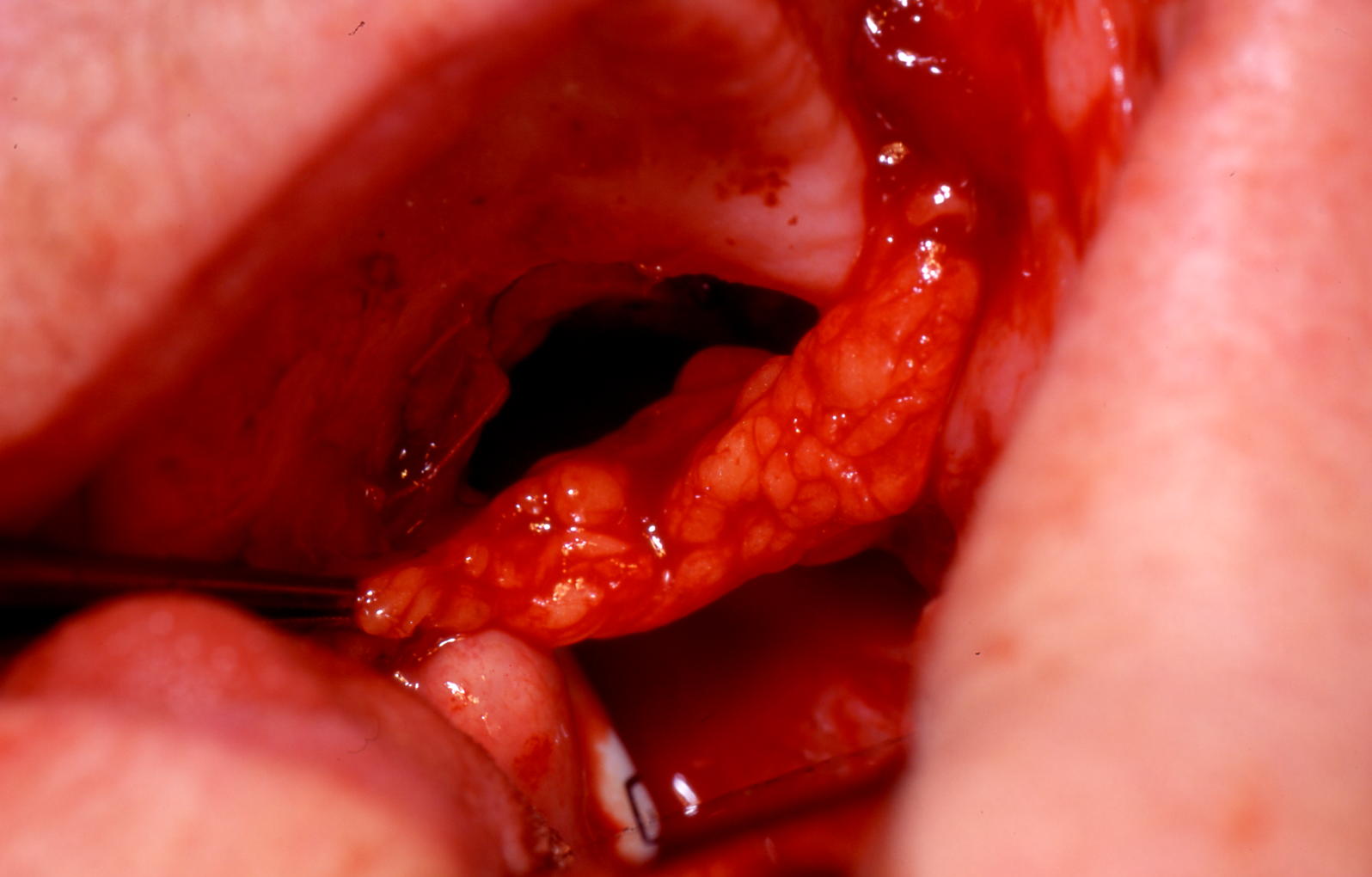
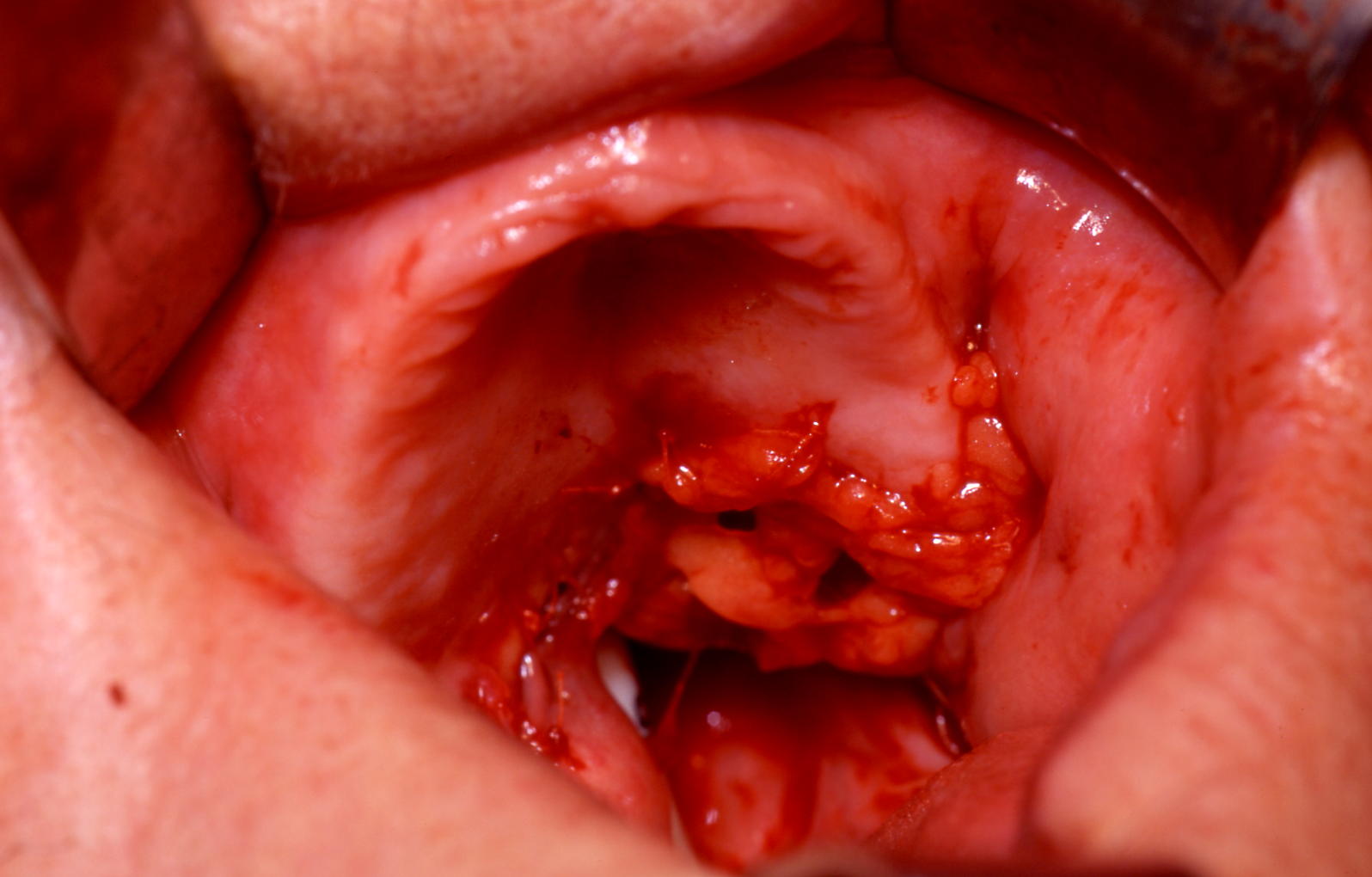
Factors to take into consideration when raising such local flaps include likely cosmetic results and ensuring the blood supply entering the flap at its base is not compromised by tension or kinking. Examples of local flaps in the oral cavity include buccal mucosa advancement flaps and the buccal fat pad flap (Figure 11 and Figure 12).
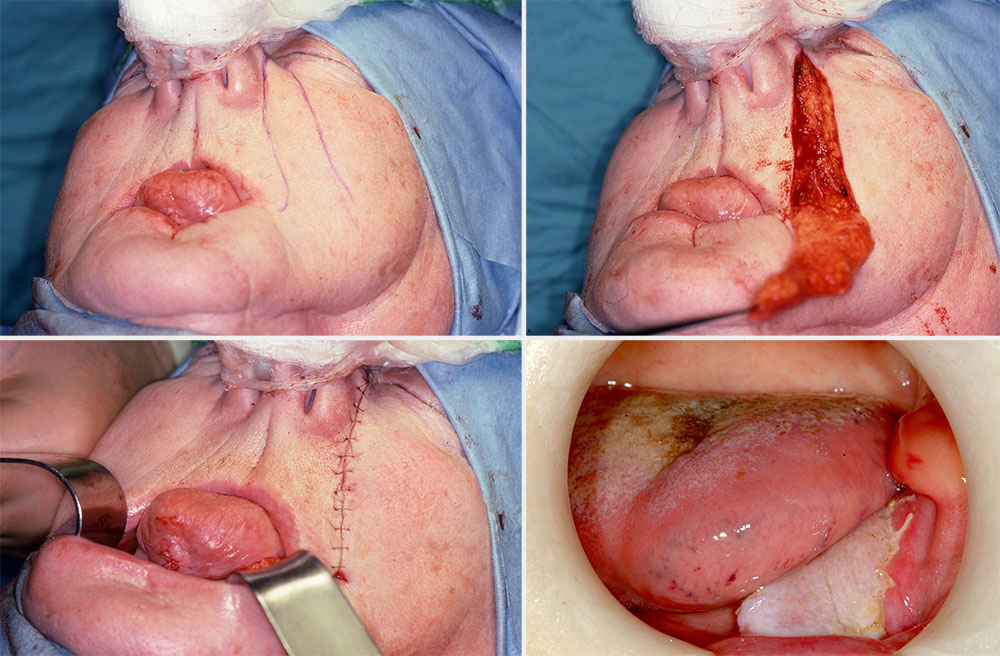
The nasolabial flap is a common example of a local pedicled flap used in the reconstruction of oral cavity defects. It is a random pattern flap and relies on the rich vascular network of the face. It is a safe reliable two-staged technique (inset to allow vascularization from the adjacent tissue bed before division of the pedicle two to three weeks later).
A finger of skin is raised (inferiorly based for oral defect) and tunnelled through the cheek and buccal mucosa to repair the adjacent floor of mouth, tongue or alveolar defects. Occasionally, the tip of the flap may necrose. Bilateral flaps are often used for small to moderate sized anterior floor of mouth defects. The donor sites are closed primarily and heal well with minimal cosmetic defect within the nasolabial folds (Figure 13).
A distant / regional pedicled flap is usually defined as any flap raised outside the local area (below the mandible) and transferred to reconstruct head and neck defects. Examples of large pedicled distant flaps used in head and neck reconstruction are the deltopectoral and the pectoralis major flaps (see below).
The deltopectoral flap is an axial patterned skin flap, based on the first four perforating branches of the internal mammary artery (Figure 14). The flap is medially based and transversely orientated on the upper chest. It is a two-staged technique, initially rotated to repair large wounds in the neck and lower part of the face. The donor site and the pedicle are temporarily dressed. At the second stage, two to three weeks later, the pedicle is divided and the proximal component reinset to the chest. The distal defect of the donor site is skin grafted (see above). This flap has been superseded by other flaps but remains a good fall-back option.
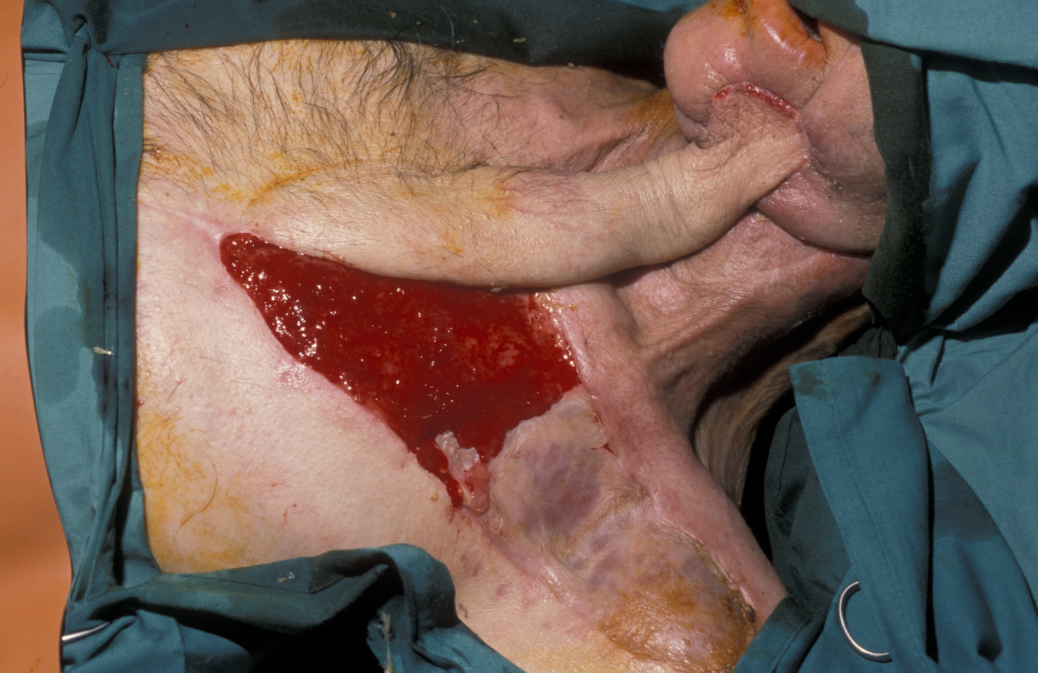
The pectoralis major flap was the ‘workhorse’ for the reconstruction of oral cavity defects in the 1980s. It remains an important reconstructive option (for example, if a patient is not fit or suitable for free flap surgery, or if no microvascular facilities or expertise are available, or if an initial free flap has failed).
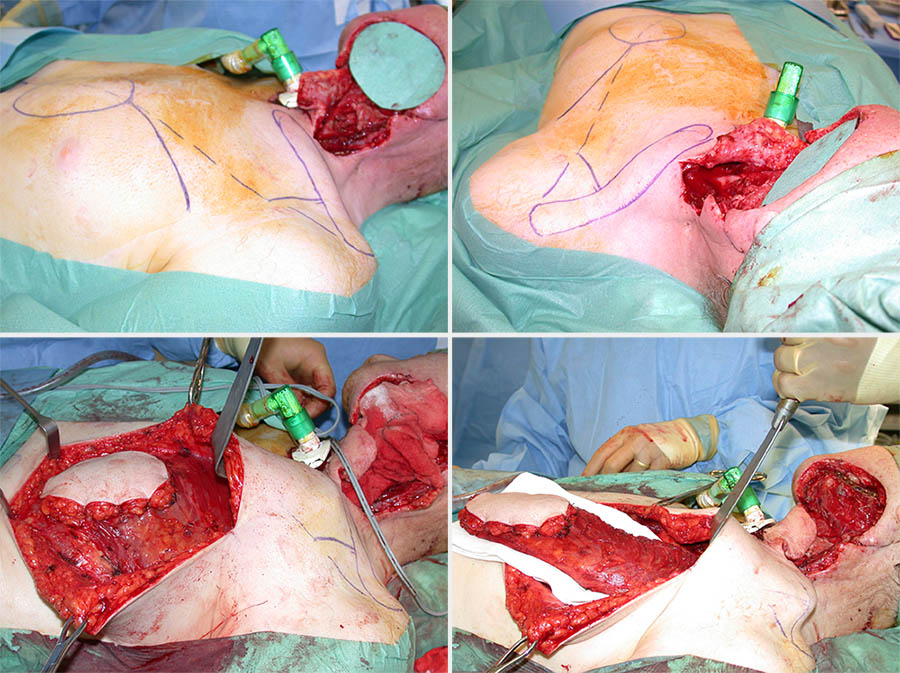
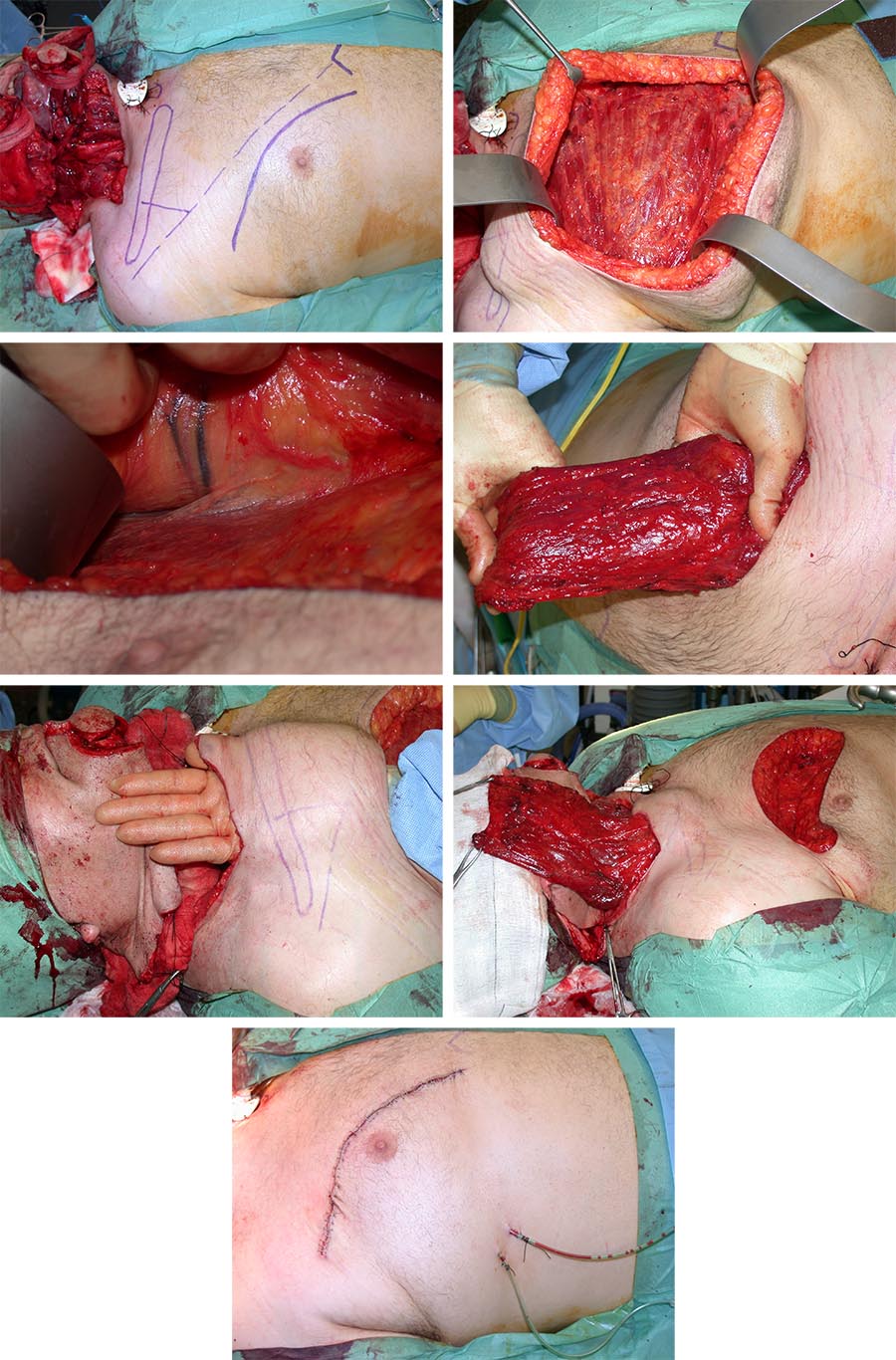
The vascular pedicle is based on the pectoral branch of the thoraco-acromial artery. It can be raised as a skin paddle (myocutaneous) or as muscle-only flap. The pectoralis major muscle is associated with a large skin territory (allowing a good sized skin paddle and usually primary closure of the donor site), good muscle bulk (useful to protect neck vessels when tunnelled into position), and a good arc of rotation enabling variable positioning for reconstruction of any head and neck defects up to the zygomatic arch. It is relatively easy to raise without the need to reposition the patient during surgery and it is generally very reliable. However, it is bulky and can be too rigid for certain oral defects requiring a more pliable reconstruction. Figure 15 shows an example of a pectoralis major myocutaneous flap, Figure 16 shows an example of a muscle-only pectoralis major flap.
Free flaps
Microvascular or free tissue transfer has been the major advancement in reconstructive surgery since the 1980s. The technique involves the harvesting and detachment of tissue with its blood (and sometimes nerve) supply and transferring it to repair a distant complex defect. The blood (and nerve) supply of the transplanted tissue is then re-established by re-anastomosis of its vascular pedicle to suitable vessels (and nerve) at the recipient site using microvascular techniques.
Microvascular surgical techniques, the ability to anastomose blood vessels using operative magnification, has revolutionized reconstructive surgery. The surgery is complex and demands certain specific processes.
- Preoperative assessment – this includes clinical examination and usually imaging (CT and/or MRI scans), for example of the donor site vascular situation. The aim is to ensure that there is no abnormal pathology or aberrant vascular anatomy that will threaten the success of flap transfer or the safety of the donor site such as viability of the foot following harvest of a fibula flap (see below).
- Perioperative issues – a two-team approach is commonly adopted, one team carrying out the ablation and the other team raising the flap simultaneously. The diameter of vessels involved commonly ranges from 1 to 3 mm. The vessels are joined end to end or end to side using 9/0 to 11/0 sutures and microsurgical instruments under an operating microscope or loupes.
- Postoperative monitoring – the flap’s viability is most at risk within the first 48 hours of surgery. Although various monitoring tools are available (including implantable Doppler (a 20 MHz pulsed ultrasound probe, directly connected with sutures to the monitored vessel on a cuff and allowing for real-time blood-flow measurement), microdialysis (monitoring flap metabolism by continuous measurements of glucose, lactate or pyruvate levels in the interstitial tissue fluids) and laser flow Doppler (optical monitoring of red blood cell flow based on Doppler effect (frequency change), using laser light) probes), regular clinical monitoring by visual inspection remains a mainstay. Venous occlusion will result in a blue engorged flap (Figure 17). Arterial blockage is more difficult to detect and results in a white flaccid flap. Flap salvage relies on prompt detection, re-exploration, identification and correction of kinking, haematoma or vascular occlusion.
There are several advantages to the use of free flaps. Complex defects are better reconstructed with a completely new blood supply (healthy fresh tissue will promote healing). Distant tissue can now be used (no longer limited by attached pedicle) and the type as well as amount of tissue harvested can be better tailored to the defect. This complex technique is now considered so reliable that it is often the first choice for reconstruction. Table 1 compares pedicled flaps to free tissue transfer.
Table 1 Comparison between pedicled and free flaps
| - | Pedicled flap | Free flap | Problem solution |
|---|---|---|---|
| Technique | Less complex | Microvascular expertise and equipment needed | Usually local expertise available |
| Duration of surgery | Shorter | Longer | Two-team approach shortens operating time |
| Reliability | Generally, more reliable but can still fail, especially partial skin necrosis | Total flap failure rate approximately 5 % | In specialized units, both flap types now reliable |
| Character of flap | Bulkier and restrictive Tissue available limited by pedicle length | Type of tissue required can be tailored better Wide tissue options available as distant sites can be transferred | |
| Donor site morbidity | Yes | Yes | See Table 2 |
| Duration of hospital stay | Perceived to be associated with less morbidity but shown to have longer hospital stays | Shorter stay unless complications develop |
There is a fair number of common free flaps used for oral defects. The radial forearm free flap is generally considered to be the workhorse for head and neck reconstruction, in particular oral soft tissue defects. It can be used for bony defects as a composite flap although other, better options exist such as the fibula, deep circumflex iliac artery (DCIA) and scapula flaps. The latter is particularly suited for complex defects as multiple tissue types at different orientations are possible based on the same vascular pedicle. The major disadvantage is the need to turn the patient mid operation. For this reason some teams use a combination of flaps that can be harvested with the patient supine.
Common flaps with their blood supply include:
- Radial forearm - radial artery.
- Pectoralis major - thoraco-acromial artery, lateral thoracic, internal mammary.
- Scapula - circumflex scapular branch of the subscapular artery.
- Latissimus dorsi - thoraco-dorsal artery.
- Anterolateral thigh - lateral circumflex femoral artery.
- Fibula - peroneal artery.
- DCIA - deep circumflex iliac artery.
- Trapezius - superiorly based: occipital artery, paraspinal perforators; lateral island: transverse cervical artery; inferiorly base: transverse cervical and dorsal scapular arteries.
- Deltopectoral - perforator arteries from the internal mammary; blood supply to the extension of this flap beyond the thoraco-acromial artery and deltopectoral groove is non-axial.
- Rectus - dual supply: superior and inferior epigastric (most flaps are raised on inferior epigastric vessels).
- Paramedian - axial flap based on the supratrochlear artery.
- Nasolabial - technically random pattern flap; the small vessels of the subdermal plexus are generally orientated along its long axis.
- Submental neck skin and platysma muscle based on the terminal branches of the facial and submental artery - useful when a neck dissection is not needed (that is for non-malignant disease).
The radial forearm free flap is a well established flap. Its skin paddle is based on the radial artery, its venae comitantes and optional cephalic vein (Figure 18).
The radial forearm flap provides soft, thin and pliable skin and fascia with, if required, bone, nerve or tendon. The quality of the skin is well suited for reconstructing intraoral defects where the aim is to provide coverage and at the same time allow residual oral tissue such as tongue and floor of mouth the flexibility to continue to function (Figure 19).
The radial forearm flap has a reliable long pedicle, good vessel caliber and allows simultaneous harvesting during head and neck ablation, reducing operating time. The donor site can be repaired with a local flap or, more commonly, with a full- or split-thickness skin graft (Figure 20).
The radial bone component is limited with regard to length and height (Figure 21) and is therefore less suitable for later rehabilitation with dental implants. The donor site radius has a significant risk of fracturing (Figure 22) although this can be overcome by prophylactic plating (Figure 23).
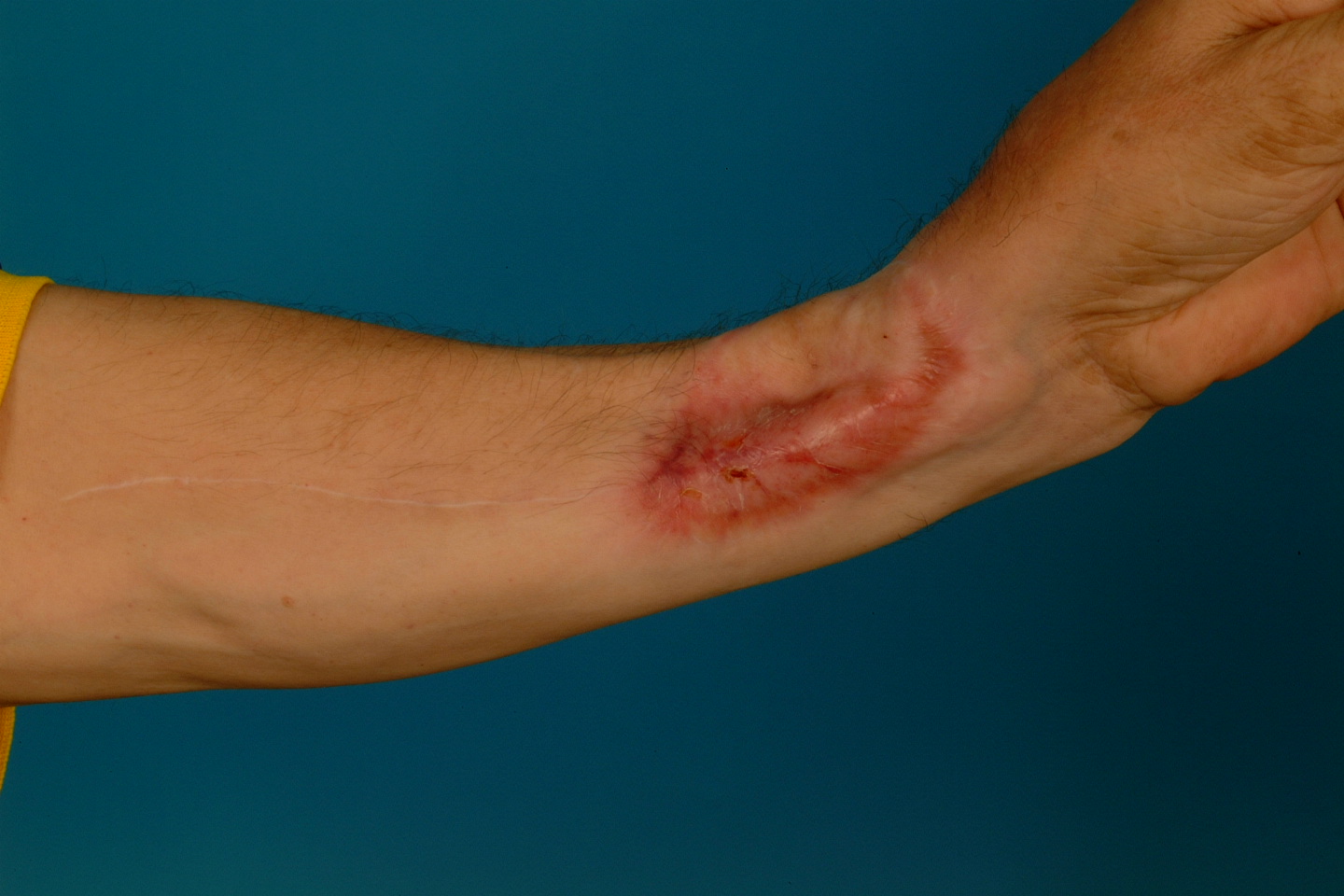
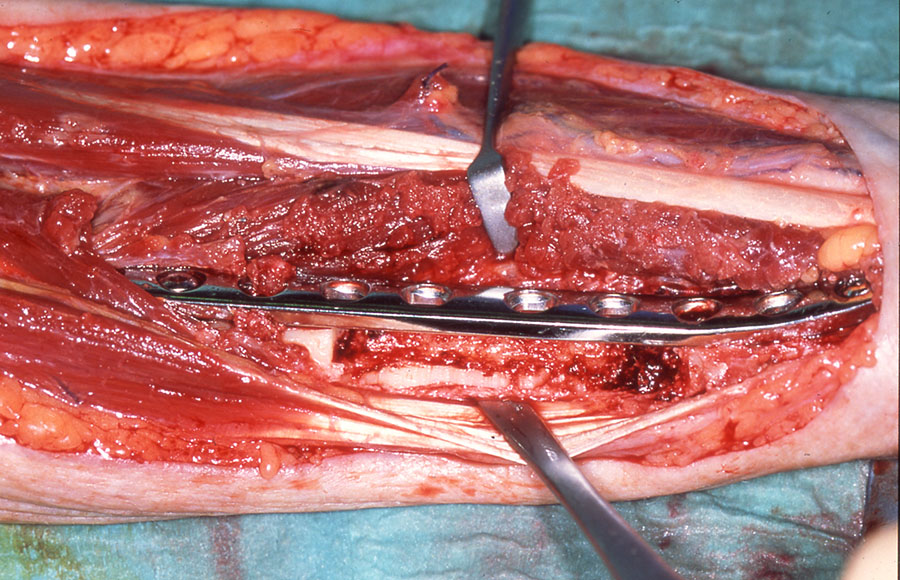
Interestingly the thin bone strut and the potential donor site defect have led to a real international polarization of views on the use of the composite radial forearm free flap. In the UK, techniques to overcome the donor site defect including prophylactic plating and full-thickness skin grafting (see above), possibly coupled with patients with very atrophic mandibles requiring composite resection, has meant the technique has been retained as part of a range of flaps for composite reconstruction. In Germany and parts of North America the technique is virtually never used and sometimes heavily criticized. Whether this is because the donor site management techniques have not been adopted or the atrophic mandible or maxilla with malignant disease is much less of an issue is unclear.
The fibula free flap provides a long (up to 25 cm), vascularized, tubular (although really more triangular) shaped, thick cortical bone capable of reconstructing an angle-to-angle mandibular defect. The flap can be raised without skin or with a skin paddle.
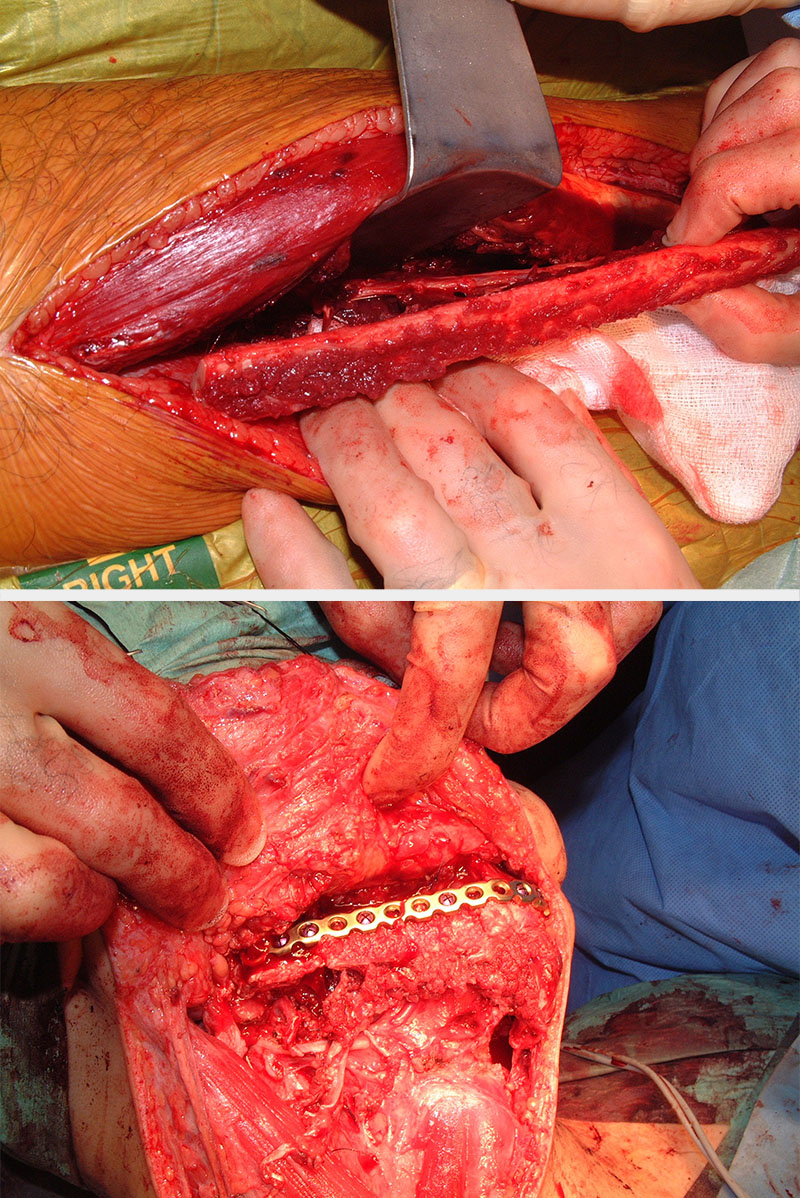
The bone is suitable for implants and, when harvested carefully, provides a reasonable skin paddle. The vascular pedicle can be lengthened significantly by trimming the proximal bone and is based on the peroneal artery and associated venae comitantes. The donor site can be primarily closed for very small skin paddles which leaves a reasonable donor site scar. The majority of skin flaps have to be split-thickness skin grafted, which delays donor site healing. Full-thickness skin grafting of this defect does not work anything like as well as it does on the forearm, and we have abandoned it.
It is important to assess the vascular supply of the donor leg. Magnetic resonance angiography, MRA is recognized internationally as the technique of choice. Duplex Doppler ultrasound assessment combined with a plain X-ray radiograph has been shown to be reliable in detecting any abnormal anatomy or pathology that may threaten the success of the flap and/or viability of the foot. Simple assessment of pulses as the only preoperative assessment, while still advocated by some, has been abandoned by most. Joint stability is not a problem if the surgeon preserves at least 6 cm of fibula bone at the knee and the ankle. There will be numbness on the dorsum of the foot and there may be some weakness in dorsiflexing the big toe.
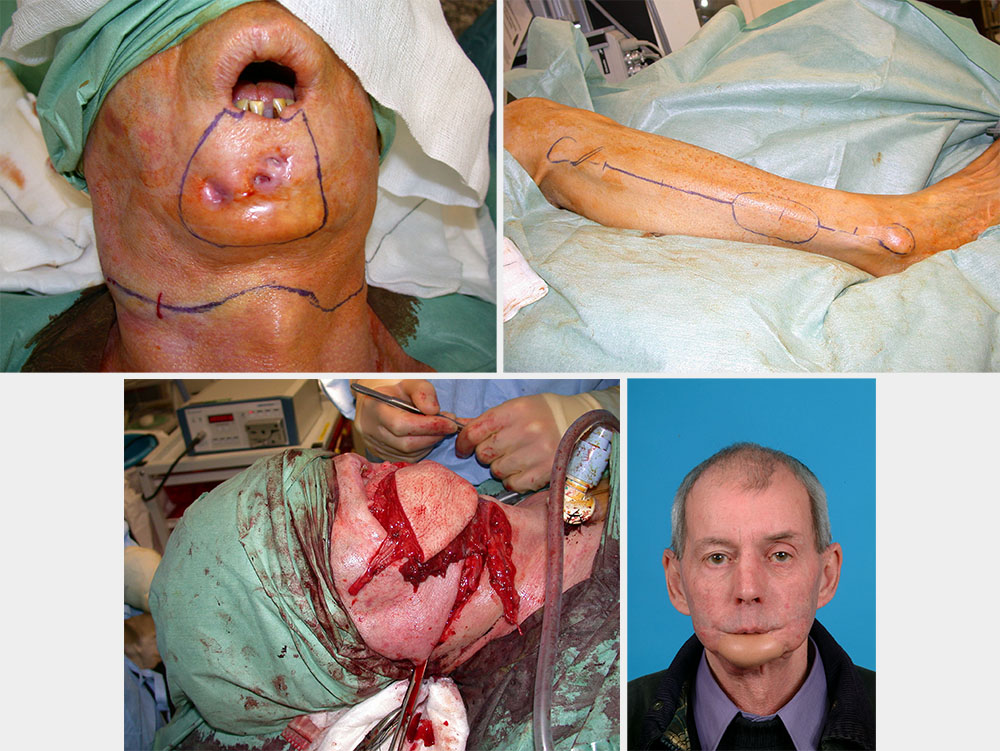
In some instances of complex defects, a single flap reconstruction is not adequate or optimal. The scapular flap with its numerous options is an excellent choice for such complex defects. However, this technique involves turning the patient peri-operatively and it increases shoulder morbidity. An alternative is to use combination flaps (Figure 25).
Certain flaps (innervated flaps) can be harvested with either their nerve supply (sensory, motor) or a vascularized nerve incorporated.
A sensate flap (for example, a radial forearm flap) can be useful if there is a recipient nerve in the head and neck such as a divided lingual nerve . Reinnervation of a sensate flap can be an advantage when reconstructing the soft palate, pharynx and lip to aid functional rehabilitation, especially with swallowing and speech . Facial reanimation is a common reason for motor reinnervation. It can also be useful to maintain muscle bulk or tone in total glossectomy.
As a working principle, it is now felt that limited sensory recovery usually occurs in thin flaps without direct nerve anastomosis and that recovery in thick flaps, even with neural anastomosis, is very limited. There is no good justification for damaging a recipient-site nerve in order to attempt sensate recovery of a flap. However, if such a nerve is resected in tumour ablation or trauma surgery, then re-anastomosis of an unavoidably sensate flap is rational. Motor nerve harvest and anastomosis follow a similar principle when reconstruction of solid tissue is the primary aim of the procedure. Facial nerve reconstruction in isolation is quite a different consideration.
In a bid to further improve the character of some free flaps, the donor site tissue planned for transfer can be modified to provide prefabricated flaps. For example, a cartilage structure harvested from the costal margin and carved to resemble a pinna (outer part of the ear) can be set into the soft tissue of a forearm distally. Once established, this modified tissue (prefabricated structure) can be transferred as a free flap to replace a missing ear. Similar procedures have been described for mucosa and bone. Inevitably these flaps are part of a delayed reconstructive process.
There remains debate about what exactly is meant by the term perforator flap. Some claim almost all flaps are ‘perforator flaps’, others list a few named flaps where visualization of the perforating vessels from the named flap vessel to the supplied tissue (usually skin) is the defining parameter. Probably the most accurate and useful definition would be to define these flaps as those where a defined area of tissue is supplied by an unnamed vessel which perforates fascia to supply skin and is identified, dissected from surrounding tissue and anastomosed when reconstructing an area with that flap. The advantage of this type of flap - no significant blood supply is impeded and only the required reconstructive tissue is harvested - would be clearly demonstrated while easily demonstrating its disadvantage: the pedicle is actually very short and the diameter is small.
Although the anterolateral thigh free flap is the most widely described ‘perforator flap’, most surgeons actually dissect the length of the descending branch of the lateral circumflex femoral vessels (thereby removing a named but relatively unimportant vascular tree). This gives a long pedicle and large diameter vessels, the actual perforating component (going through fascia to supply skin) is a relatively short length of vessels requiring intramuscular dissection in 80 % of patients. The skin paddle of the fibula often has visible perforating vessels to the skin, but these are seldom dissected through muscle and fascia to isolate them. The lateral sural artery perforator flap is variably dissected as a soleal branch of the peroneal vessels and the medial sural artery perforator flap always involves intramuscular dissection – all short expendable vessels but they are anatomically named. The relative significance of retaining the tissue (usually muscle) at the donor site is very debatable, with the possible exception of the transverse deep inferior epigastric perforator flap used for breast reconstruction in younger women or for soft tissue trauma coverage, where there is an arguable advantage in retaining a semi-functioning rectus abdominus muscle.
Outcomes
Free flap success rates are usually reported to be in excess of 95 %. Inevitably, there are local variations with types of flap and expertise available. The presence of co-existing medical conditions (usually cardiorespiratory diseases, expected in smokers) can have an important bearing on the likelihood of complications, which in turn can affect viability of free flaps during the immediate postoperative stage.
A free flap technique offers many advantages but is an all or none phenomenon. Total flap loss will result in a persisting defect and a weakened patient. A second reconstructive procedure can put that patient at a significant risk. Donor site morbidity should always be considered during the treatment planning as this is of considerable importance to overall quality of life.
Factors affecting free flap success include:
local factors such as
- surgical mishaps (such as microsurgical suturing, pedicle tension or compression)
- wound complications (such as haematoma, bleeding or wound infection;
general factors such as
- systemic hypotension with inadequate arterial flow;
- increased coagulability (excessive transfusion, donor site bleeding);
- vascular spasm (cold environment, smoking).
Before moving on and discussing reconstructive options for different head and neck defects on the following page, we give a brief overview of various soft and hard tissue flaps in Table 2.
Table 2 Strengths and weaknesses of common free flaps in head and neck reconstruction
| Flap and its common usage | Strengths | Drawbacks and morbidity |
|---|---|---|
| Soft tissue flaps | ||
| Radial forearm Widely used for oral defects |
Good supple skin paddle. Reliable long vascular pedicle. Can be harvested simultaneously with ablation (two-team approach). | Scar visible on forearm. Skin graft can be lost. Numbness in superficial radial nerve distribution. |
| Lateral
arm Reasonable alternative to radial forearm free flap |
Scar can be closes primarily and is easier to hide. | Risk to radial nerve. Shorter pedicle. Difficult to harvest st same time as head and neck ablation. |
| Latissimus
dorsi Large soft tissue paddle |
Large soft tissue flap. Reliable vessels, Can be used with scapular system. Primary closure, hidden scar. | Patient requires turning during surgery. Winging of scapula. Poor colour match to face. |
| Anterolateral
thigh Large skin paddle |
Large soft tissue paddle with optional adjacent fascia lata and muscle. Allows two-team approach. Primary closure, hidden scar. | Skin perforators can be tenuous. Poor colour match to face. |
| Rectus abdominis Very large soft tissue coverage |
Large soft tissue paddle with rectus sheath. Allows two-team approach. Hidden scar. | Hernia. Abdominal wall weakness. Poor colour match to face. |
| Bone flaps | ||
| Radial forearm
composite Large edentulous defects with large soft tissue defects |
Good skin paddle. Long pedicle and reliable vessels. Allows two-team approach. | Poor bone stock. Radial fracture risk (prophylactic plating willover come this). |
| Fibula Long bony defects |
Long bone (up to 25 cm). Good vascular pedicle. | Skin paddle needs careful harvesting. Skin graft to donor site required for larger skin paddles. Risk of vascular compromise to foot. Risk of weak dorsiflexion of big toe, numbness on foot. |
| Deep circumflex iliac
crest Large bone stock for implantation |
Bone easy to contour. Best bone stock for implantation. Allows two-team approach. | Wide dissection and difficult in obese patients. Shorter pedicle, bulky skin paddle. |
| Scapula Complex defects with bone |
Allows separate skin paddles and bone. | Need to turn patient during surgery. Two-team approach difficult. Shoulder disability risk. |